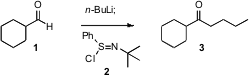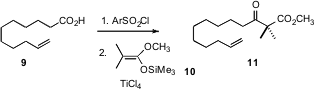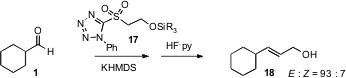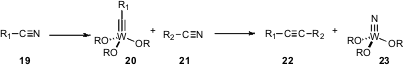Carbon-carbon bond construction is the basis for all of organic chemistry. Important methods for the construction of single, double, and triple bonds have been described.
While ketones can be prepared by the addition of organometallic reagents to amides or to nitriles, they are often prepared by the addition of the organometallic reagent to an aldehyde such as 1. In a subsequent step, the product secondary alcohol is oxidized to the ketone. William J. Kerr of the University of Strathclyde has now found (Org. PMID:24278086 Lett. 2006, 8, 5073. DOI: 10.1021/ol061903l)that if the intermediate alkoxide is worked up with the Mukaiyama reagent 2, the ketone3 is formed directly. The alkoxides derived from the addition of Grignard reagents can be converted to the ketones, but yields are better with the alkoxides derived from organolithium reagents.
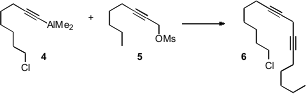
Skipped diynes such as 6 are often prepared as precursors to the skippedZ-dienes that are ubiquitous in fatty acids. Formula of 4693-47-4 (S)-4-Oxopyrrolidine-2-carboxylic acid Purity Sebastian Wendeborn of Syngenta Crop Protection in Basel has developed (Org. Lett. 2006, 8, 5629. DOI: 10.1021/ol0623769)a new route to such skipped diynes, based on the coupling of an alkynylalane 4 with a propargylic methanesulfonate. The regioisomeric allenic products are apparently not formed.
The aldol condensation of esters with aldehydes has required the formation of the stoichiometric ester enolate. Don M. Coltart of Duke University has now shown (Org. Lett. 2006, 8, 1503. DOI: 10.1021/ol060413q)that Hunig’s base in the presence of MgBr2 etherate is sufficient to induce the condensation of a thioester such as 7 with the aldehyde 1. Note that the product thioester 8 could easily be reduced to the aldehyde or homologated to the ketone.
In a continuation of his work on innovative and practical procedures for acylation, Yoo Tanabe of Kwansei Gakuin University, Hyogo, has described (Org. Lett. 2006, 8, 5215. DOI: 10.1021/ol0619361)the activation of a carboxylic acid such as 9 by formation of the mixed anhydride, followed by condensation with a ketene silyl acetal 10, to give the β-keto ester 11.
KH is a fast and efficient base, but it has been used far less in organic synthesis than it might have been, because it comes as a slurry in mineral oil, and so is difficult both to handle and to dispense precisely in small quantities. We have found (J. Org. Chem. 2006, 71, 8973. DOI: 10.1021/jo061420v)that KH can be washed clean, then suspended in melted paraffin wax. The cooled wax, KH(P), 50% by weight KH, is easy to dispense, and is stable in air for months at least.
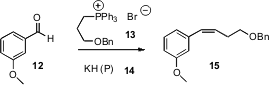
Allylic alcohols are ubiquitous intermediates in organic synthesis. Often, these are prepared by Horner-Emmons homologation of an aldehyde such as 1, followed by reduction. István E. Markó of the Université catholique de Louvain has now developed (Org. Lett. 2006, 8, 5983. DOI: 10.1021/ol062433y)a practical alternative, Kocienski-Julia homologation using 17. If the silyl-protected allylic alcohol is desired, the HF•pyridine step can be omitted.
Methods for the direct preparation of C-C triple bonds are limited. Marc J. A. Johnson of the University of Michigan, in the course of work directed toward catalytic C-C triple bond formation (J. Am. Chem. Soc. 2006, 128, 9614. DOI: 10.1021/ja058036k), reminds us that the sequential tungsten-mediated stoichiometric coupling of two nitriles works well. This could be a practical route to high value-added alkynes 22.