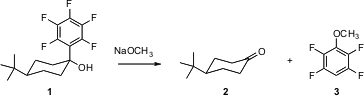
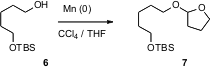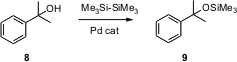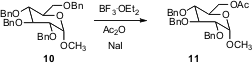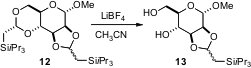Charles M. Garner of Baylor University has described (Tetrahedron Lett. 2006, 47, 7405. DOI: 10.1016/j.tetlet.2006.08.069)the fragmentation of alcohols such as 1 to give the ketone2. The alcohols are prepared by the addition of pentafluorophenyl magnesium bromide to the ketone, so this is a method for ketone protection and deprotection.
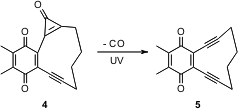
Vladimir V. Popik, now at the University of Georgia, has observed (J. Org. Formula of 4-(6-Bromopyridin-3-yl)morpholine Chem. 2006, 71, 7417. DOI: 10.1021/jo061285m)that UV light effficiently converts the cyclopropenone3 into the diyne 4. The cyclopropenone is prepared by the addition of dichlorocarbene to the alkyne, followed by hydrolysis. This approach allows the alkyne to be released under mild, controlled conditions. PMID:23849184 178432-48-9 uses
Tetrahydrofuranyl is a good protecting group for alcohols, but it has been little used. J. R. Falck of UT Southwestern Medical Center and Charles Mioskowski of the Université Louis Pasteur, Illkirch have devised (Tetrahedron Lett. 2006, 47, 5111. DOI: 10.1016/j.tetlet.2006.05.081)a convenient procedure for attaching this protecting group, using Mn powder. Primary, secondary, and tertiary alcohols are easily protected using this procedure.
TMS ethers of hindered alcohols such as 8 are usually prepared using the unstable and corrosive TMS triflate. Eiji Shirakawa and Tamio Hayashi of Kyoto University have now found (Chem. Commun. 2006, 3927. DOI: 10.1039/b608958e)that Pd catalyzes the transfer of both silyl groups from the easily handled Me3Si-SiMe3. Again, primary, secondary and tertiary alcohols work well.
Benzyl ethers are usually removed by hydrogenation or by dissolving metal reduction. Yashwant D. Vankar of the Indian Institute of Technology, Kanpur has developed (Tetrahedron Lett. 2006, 47, 5207. DOI: 10.1016/j.tetlet.2006.05.016)an alternative, BF3 ethereate in the presence of acetic anhydride and NaI. Silyl ethers are also converted to the corresponding acetates under the reaction conditions.
The selective protection of 1,2- and 1,3-diols is also important. To this end, Jun’ichi Uenishi of Kyoto Pharmaceutical University has prepared (Tetrahedron Lett. 2006, 47, 5553. DOI: 10.1016/j.tetlet.2006.05.121)the dimethyl acetal of triisopropylsilyl acetaldehyde. The derived cyclic acetals, such as 12, are orthogonal to acetonides – each can be selectively removed in the presence of the other.
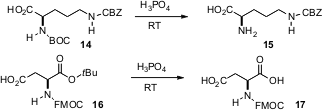
There have been several important developments in N protection. BOC groups are usually removed with trifluoroacetic acid. Bryan Li of Pfizer in Groton, CT has now shown (J. Org. Chem. 2006, 71, 9045. DOI: 10.1021/jo061377b)that commericial, environmentally benign 85% phosphoric acid at room temperature works equally well. t-Butyl esters are also cleaved. Mona Hosseini-Sarvari and Hashem Sharghi of Shiraz University, Iran have found (J. Org. Chem. 2006, 71, 6652. DOI: 10.1021/jo060847z)that ZnO with formic acid serves to formylate amines. Toshio Nishikawa and Minoru Isobe of Nagoya University have developed a mild protocol (Org. Lett. 2006, 8, 3263. DOI: 10.1021/ol061123c)for converting trichloroamides (from trichloroacetimidate rearrangement) into readily deprotectable carbamates. Floris P. J. T. Rutjes of Radboud University, Nijmegen has found (Tetrahedron Lett. 2006, 47, 8109. DOI: 10.1016/j.tetlet.2006.09.044)that the inexpensive trichloroisocyanuric acid serves to efficiently dearylate p-methoxyphenyl amines.