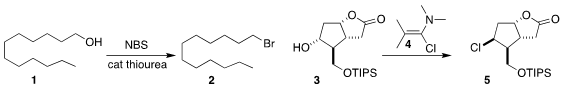
N. Gabriel Lemcoff of the Ben-Gurion University of the Negev and Ofer Reany
of the Open University of Israel used a thiourea to catalyze the
N-bromosuccinimide conversion of the alcohol
1 to the bromide 2
(J. Org. PMID:28739548 Chem. 2020, 85, 12901.
DOI: 10.1021/acs.joc.0c01431).
Léon Ghosez of the University of Bordeaux and UCLouvain showed that the conversion of the alcohol 3
to the chloride 5 with the chloro enamine
4 proceeded with clean inversion of absolute configuration
(Tetrahedron 2020, 76, 131441.
DOI: 10.1016/j.tet.2020.131441).
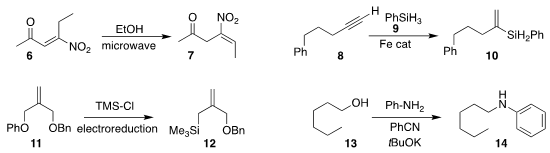
Alessandro Palmieri of the University of Camerino observed that the deconjugation of the enone
6 to the nitroalkene 7 proceeded with high geometric selectivity
(Adv. Synth. Catal. 2020, 362, 4680.
DOI: 10.1002/adsc.202000747).
Shou-Fei Zhu of Nankai University demonstrated that with the proper choice of iron catalyst, phenyl
silane 9 could be added to the alkyne 8 to give either regioisomer of the
alkenyl silane 10
(J. Am. 3-Methylcyclopentane-1-carboxylic acid Order Chem. Soc. 2020, 142, 16894.
DOI: 10.1021/jacs.0c09083).
Song Lin of Cornell University prepared the
allyl silane 12 by the reductive coupling of the phenyl
ether 11 with trimethylsilyl chloride
(J. 3,4-Diaminobenzenesulfonic acid Chemical name Am. Chem. Soc. 2020, 142, 21272.
DOI: 10.1021/jacs.0c10899).
Bitragunta Sivakumar and Subramaniyan Mannathan of SRM University-AP used
benzonitrile to mediate the
N-alkylation of aniline
with the alcohol 13
(Adv. Synth. Catal. 2020, 362, 4409.
DOI: 10.1002/adsc.202000499).
Chunyan Zhang and Guoyin Zhang of the Qingdao
University of Science and Technology and Mao-Lin Hu of Wenzhou University
described a parallel investigation
(Chem. Commun. 2020, 56, 10489.
DOI: 10.1039/D0CC04831C).
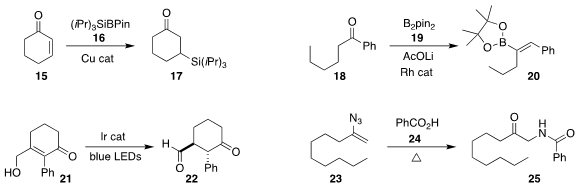
Koji Kubota and Hajime Ito of Hokkaido University developed a general route
to the trialkylsilylborane 16, and added it in a conjugate sense to
cyclohexenone
15, leading to the ketone 17
(J. Am. Chem. Soc. 2020, 142, 14125.
DOI: 10.1021/jacs.0c03011).
Zhenyang Lin of Hunan University and Wanxiang Zhao of the Hong Kong University
of Science and Technology used a Rh catalyst to add B2pin2 (19) to the ketone
18, leading to the
alkenyl boronate 20
(J. Am. Chem. Soc. 2020, 142, 18118.
DOI: 10.1021/jacs.0c07854).
Jun Huang and Zhen Yang of Peking University Shenzhen Graduate School used an iridium photocatalyst
to mediate the isomerization of the allylic alcohol 21 to the aldehyde 22
(Angew. Chem. Int. Ed. 2020, 59, 11660.
DOI: 10.1002/anie.202000743).
Xinying Zhang and Xuesen Fan of Henan Normal University assembled the amide 25
by coupling benzoic acid (24) with the alkenyl azide 23
(J. Org. Chem. 2020, 85, 13710.
DOI: 10.1021/acs.joc.0c01871).
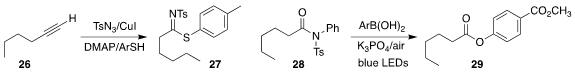
Chien-Fu Liang of National Chung Hsing University prepared the
N-sulfonylthioimidate 27 from the terminal alkyne 26
(Org. Biomol. Chem. 2020, 18, 8881.
DOI: 10.1039/D0OB01963A).
Qiuping Ding of Jiangxi Normal University and Yong Luo of Sun Yat-Sen
University established oxidative conditions for the conversion of the N-sulfonyl
amide 28 to the aryl ester 29
(Tetrahedron Lett. 2020, 61, 152444.
DOI: 10.1016/j.tetlet.2020.152444).
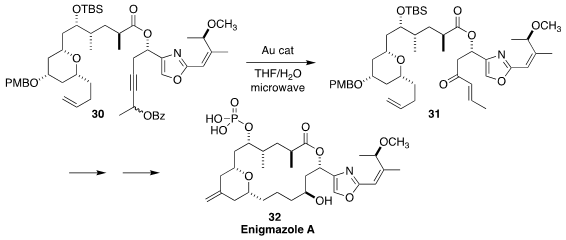
Enigmazole A (32), isolated from a marine sponge Cinachyrella enigmatica of
Papua New Guinea, showed activity in the NCI 60-cell panel. In the course of a
synthesis of 32, Haruhiko Fuwa of Chuo University used a gold catalyst to effect
the rearrangement of the propargyl ester 30 to the enone 31
(Chem. Asian J. 2020, 15, 3494.
DOI: 10.1002/asia.202001015).