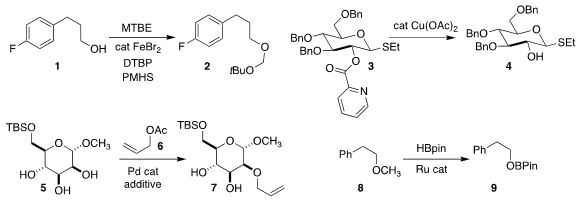
Wei Han of Nanjing Normal University used oxidative conditions to protect the
alcohol of 1 as the mixed acetal 2
(Synlett 2020, 31, 1400.
DOI: 10.1055/s-0040-1707162).
Alexei V. Methyl 3-(1H-pyrrol-2-yl)propanoate site Demchenko of the University of Missouri, St. Louis devloped a protocol
for removing the picoloyl ester from 3, leading to 4
(Org. Biomol. Chem. PMID:28739548 2020, 18, 4863.
DOI: 10.1039/D0OB00803F).
Dawen Niu of Sichuan University demonstrated that with the addition of the appropriate additive,
the triol 5 could be coupled with allyl acetate 6 to give each of the three possible
monoallyl ethers, illustrated by 7
(Nature Commun. 2020, 11, 5681.
DOI: 10.1038/s41467-020-19348-x).
Chidambaram Gunanathan of the National Institute of
Science Education and Research devised conditions for converting the methyl
ether 8 selectively to the boronate ester 9, with loss of methane
(ACS Catal. 2020, 10, 14390.
DOI: 10.1021/acscatal.0c04269).
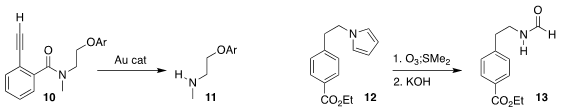
Kenward Vong and Katsunori Tanaka of RIKEN designed the 2-alkynylbenzamide
10, that could be deprotected to the amine 11 by exposure to a gold catalyst
(Chem. Sci. 916304-19-3 Data Sheet 2020, 11, 10933.
DOI: 10.1039/D0SC04329J).
Paul Knochel of Ludwig-Maximilians-Universität
München ozonized the N-alkyl pyrrole
12 to give the formamide 13
(Chem. Eur. J. 2020, 26, 8951.
DOI: 10.1002/chem.202000870).
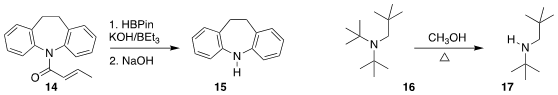
Wubing Yao of Taizhou University reduced the amide 14 with
HBpin,
leading to the amine 15
(Org. Lett. 2020, 22, 8086.
DOI: 10.1021/acs.orglett.0c03033).
Klaus Banert of the Chemnitz University of Technology, who unfortunately passed away in 2020,
and Paul R. Rablen of Swarthmore College found that the highly congested tertiary amine 16 underwent
Hofmann-like
elimination to the corresonding secondary amine 17 at only slightly elevated temperature
(J. Org. Chem. 2020, 85, 13630.
DOI: 10.1021/acs.joc.0c01790).
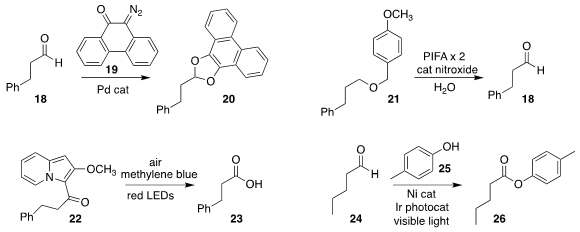
Mitsuru Kitamura of the Kyushu Institute of Technology used the α-diazoketone
19 to protect the aldehyde 18 as the acetal 20
(Eur. J. Org. Chem. 2020, 5319.
DOI: 10.1002/ejoc.202000315).
Shohei Hamada of the Kyoto Pharmaceutical University oxidized the
primary p-methoxybenzyl ether 21 directly
to the aldehyde 18
(Org. Lett. 2020, 22, 5486.
DOI: 10.1021/acs.orglett.0c01839).
Kenji Watanabe and Takamitsu Hosoya, also of RIKEN, used long wavelength
visible light to convert the aryl ketone 22
to the carboxylic acid 23
(Org. Lett. 2020, 22, 5434.
DOI: 10.1021/acs.orglett.0c01799).
Naoki Ishida and Masahiro Murakami of Kyoto University also used visible light to mediate the
oxidative coupling of the aldehyde
24 with the phenol 25, leading to the aryl ester 26
(Angew. Chem. Int. Ed. 2020, 59, 18267.
DOI: 10.1002/anie.202008897).
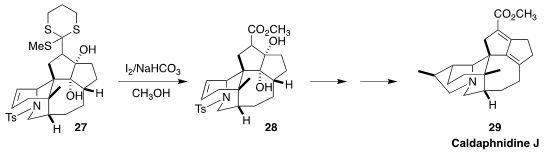
Caldaphnidine J (29) is the first of the yuzurimine subgroup of the
Daphniphyllum alkaloids to be prepared. In the course of this synthesis, Jing Xu
of the Southern University of Science and Technology converted the α-sulfinyl
dithiane 27 to the methyl ester 28
(Nature Commun. 2020, 11, 3538.
DOI: 10.1038/s41467-020-17350-x).