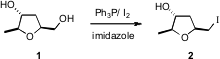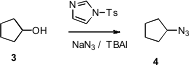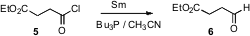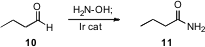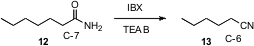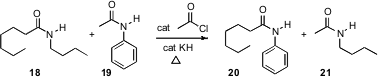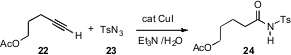Both John Boukouvalas of the Université Laval (Tetrahedron Lett. PMID:22664133 2007, 48, 2971. 5-Bromo-1H-pyrazole-3-carboxylic acid In stock DOI: 10.1016/j.tetlet.2007.03.014)and Leo A. Paquette of the Ohio State University (Org. Lett. Buy5-Oxaspiro[2.4]heptane-1-carboxylic acid 2007, 9, 719. DOI: 10.1021/ol063083i)have found that the well-established I2/Ph3P/imidazole protocol for conversion of alcohols to iodides is primary selective, so 1 was converted smoothly into 2.
The conversion of alcohols to azides is also well established. Mohammad Navid Soltani Rad of Shiraz University of Technology has developed (Tetrahedron Lett. 2007, 48, 3445. DOI: 10.1016/j.tetlet.2007.03.049)a mild new reagent combination for effecting this transformation. Iodide is a component of the reaction mixture, so displacement of a secondary benzylic alcohol proceeded with partial racemization. The stereochemical outcome with isolated secondary alcohols was not reported.
Both hydrogenation (Rosenmund) and hydride have been used to reduce acid chlorides to aldehydes. Xueshun Jia of Shanghai University has shown (Tetrahedron Lett. 2007, 48, 971. DOI: 10.1016/j.tetlet.2006.12.014)that Sm metal also effects this transformation. Nitro groups and alkenes were stable to the conditions.
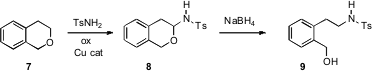
Methods for C-H functionalization are well developed. Xiao-Qi Yu of Sichuan University has taken advantage (Org. Lett. 2007, 9, 2277. DOI: 10.1021/ol070537i)of easy C-H amination to convert the ether 7 to the sulfonamide 8. Reduction then delivered the hydroxy sulfonamide 9.
Conversion of an aldehyde to a primary amide would usually take three steps (acid, activation, amide). Jonathan M. J. Williams of the University of Bath has developed (Org. Lett. 2007, 9, 73. DOI: 10.1021/ol062549u)an Ir catalyst that, in the presence of hydroxylamine, can effect the transformation of 10 to 11 in a single step.
Degradation reactions, reactions that remove carbons, can also be useful. Krishnacharya G. Akamanchi of the University Institute of Chemical Technology, Mumbai has found (J. Org. Chem. 2007, 72, 662. DOI: 10.1021/jo0619074)that IBX converts primary amides such as 12 to the nitrile 13, with loss of one carbon atom. This reaction is apparently proceeding via N-bromination followed by rearrangement to the isocyanate.
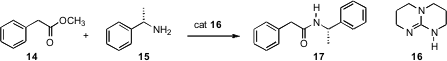
There have been several other interesting developments in the synthesis of amides. Charles Mioskowski of the Université Louis Pasteur de Strasbourg has uncovered (Tetrahedron Lett. 2007, 48, 3863.DOI: 10.1016/j.tetlet.2007.03.146)a useful catalyst16 for the condensation of esters with amines to form amides.
Samuel H. Gellman and Shannon S. Stahl of the University of Wisconsin have devised (Angew. Chem. Int. Ed. 2007, 46, 761.DOI: 10.1002/anie.200603588)a simple procedure for amide metathesis. Use of an excess of 19 would be expected to drive the 18 to 20 conversion to completion.
Anti-Markovnikov hydration of a terminal alkyne yields the corresponding carboxylic acid. Sukbok Chang of KAIST, Daejon has reported (Angew. Chem. Int. Ed. 2007, 46, 1897.DOI: 10.1002/anie.200604358)that CuI is an effective catalyst for the combination of a terminal alkyne 22 with a sulfonyl azide 23 to give the amide 24. Internal alkynes are stable to the reaction conditions.
Unfortunately, Professor Charles Mioskowski passed away in June. This column is dedicated to his memory.