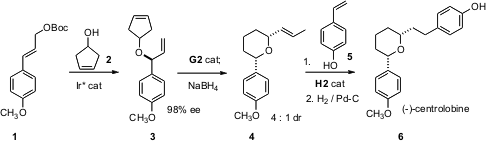
The recent (Chem. Commun. 2006, 1968.DOI: 10.1039/b602056a)synthesis of (-)-centrolobine (6) by Siegfried Blechert of the Technische Universität Berlin is a tour de force of organometallic catalysis. The absolute configuration of the natural product was set by the Ir*-mediated SN2′ coupling of 1 and 2. Diastereoselective ring closure with the second-generation Grubbs catalyst was terminated by the addition of NaBH4, to reduce the Ru catalyst to a Ru-H species, which mediated the migration of the terminal alkene that was the initial metathesis product. Cross metathesis with5 using the second-generation Hoveyda catalyst followed by catalytic hydrogenation then delivered 6. The second-generation Hoveyda catalyst is sometimes termed the “Hoveyda-Grubbs” catalyst.
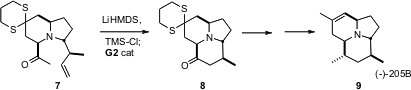
The cyclization of 7 to 8 was a key step in the synthesis (J. Price of Methyl 5-bromo-4-iodonicotinate Org. PMID:23935843 Chem. Formula of 8-Fluoro-1,2,3,4-tetrahydroquinoline 2006, 71, 2547.DOI: 10.1021/jo052314g)of the Dendrobatid alkaloid (-)-205B (9) by Amos B. Smith III of the University of Pennsylvania. In this reaction, which was based on the precedent of Masakatsu Shibasaki of the University of Tokyo (Tetrahedron Lett. 2001, 42, 8023.DOI: 10.1016/S0040-4039(01)01713-0)the silyl enol ether derived from 7 underwentring-closing metathesis, followed by hydrolysis of the product enol ether.
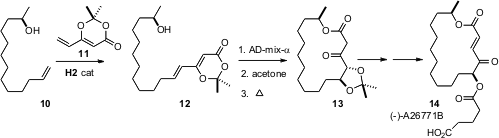
In what promises to be a general approach to macrolactones, Professor Blechert has prepared (J. Org. Chem. 2006, 71, 2021.DOI: 10.1021/jo052421a)the diene lactone 11. Cross metathesis with the unsaturated alcohol 10 led to 12, which after dihydroxylation and protection was heated to generate the macrolactone 13, by way of the intermediate ketene. The acetonide 13 was carried on the macrolide antibiotic (-)-A26771B.
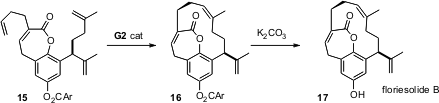
The macrolide floreside (17) is in fact a [10]metacyclophane, further bridged by the lactone ring. This led to the question of which ring to form first. K. C. Nicolaou of Scripps, La Jolla found (Chem. Commun. 2006, 600. DOI: 10.1039/b517385j)that cyclophane formation was reluctant under metathesis conditions, but that if the lactone was formed first, the second-generation Grubbs catalyst smoothly closed the carbocyclic ring. The product 16 was formed as a single atropisomer, corresponding to the natural product 17.
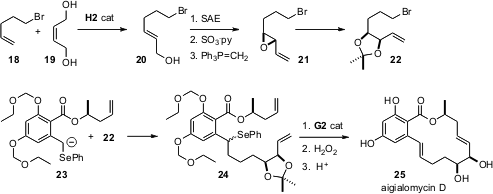
In a modular synthesis of the macrolide aigialomycin D (25) (Angew. Chem. Int. Ed. 2006, 45, 3951.DOI: 10.1002/anie.200600593), Nicolas Winssinger of the Université Louis Pasteur in Strasbourg prepared the substrate 20 forSharpless asymmetric epoxidation using the method that we introduced (J. Org. Chem. 2003, 68, 6047.DOI: 10.1021/jo030005p), equilibrating homologation with the inexpensive 2-butene-1,4-diol (19). Acetonide formation from 21, with single inversion, then delivered the alkylating agent 22. The selenide of 23 enabled anion formation, and also served as a masked alkene. The authors observed that it was important to effect ring-closing metathesis before the oxidative elimination.