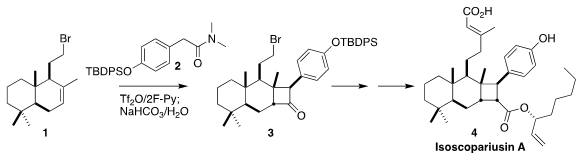
Léon Ghosez demonstrated that [2+2] cycloaddition was particularly efficient
with keteniminum salts. Ang Li of the Shanghai Institute of Organic Chemistry
and Pema-Tenzin Puno of the Kunmin Institute of Botany extended this to the
dehydrative addition of the amide 2 to the congested alkene 1,
leading, after hydrolysis, to the
cyclobutanone
3, that they carried on to isoscopariusin A (4)
(Angew. Chem. Int. PMID:23522542 Formula of 751470-47-0 Ed. 2021, 60, 12859.
DOI: 10.1002/anie.202100288).
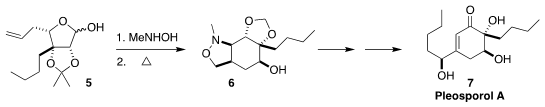
Pleosporol A (7), isolated from the marine fungus Pleospora sp,
showed marked antimicrobial activity. 6-Fluorobenzofuran-2-carboxylic acid uses Jinzhong Xu of Zhejiang University,
Zhoushan assembled the highly substituted
cyclohexane core 6
by the combination of the lactol 5 with N-methylhydroxylamine followed by heating
(Tetrahedron 2021, 81, 131913.
DOI: 10.1016/j.tet.2020.131913).
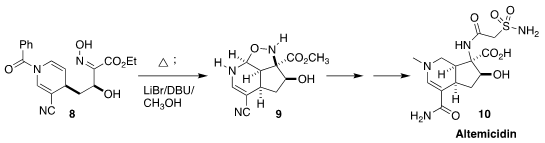
Thomas J. Maimone of the University of California, Berkeley also used intramolecular
dipolar cycloaddition
to construct the carbocyclic core of
altemicidin 10. Heating of the oxime 8 followed by direct
methanolysis yielded the crystalline nitrile 9
(J. Am. Chem. Soc. 2021, 143, 7935.
DOI: 10.1021/jacs.1c04147).
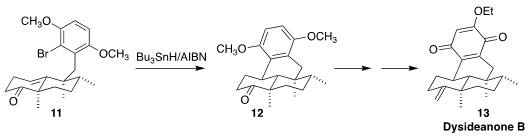
Dysideanone B (13), isolated from the South China Sea sponge
Dysidea avara, showed marked cytotoxicity. In the course of a synthesis of
13, Zhaoyong Lu of Nankai University observed high diastereoselectivity
in the radical cyclization of the bromoarene 11 to the ketone 12
(Angew. Chem. Int. Ed. 2021, 60, 13807.
DOI: 10.1002/anie.202100541).
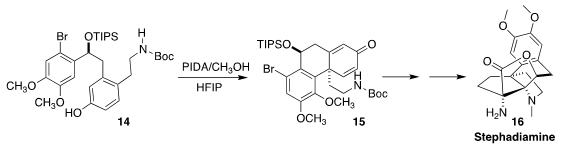
Stephadiamine (16) was isolated from the Stephania japonica, the snake
vine of Southeast Asia. En route to 16, Minami Odagi and Kazuo Nagasawa
of the Tokyo University of Science and Technology achieved high
diastereoselectivity in the oxidative cyclization of the secondary silyl ether
14 to the cyclohexadienone 15
(J. Am. Chem. Soc. 2021, 143, 2699.
DOI: 10.1021/jacs.1c00047).
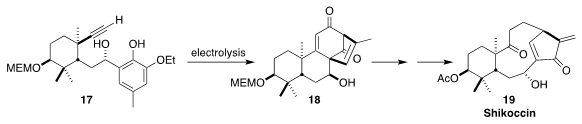
Shikoccin (19) was isolated from the Chinese medicinal herb
Rabdosia shikokiana. Hanfeng Ding of Zhejiang University constructed the
tetracyclic intermediate 18 by the oxidative electrolysis of the alkynyl
arene 17
(Angew. Chem. Int. Ed. 2021, 60, 14892.
DOI: 10.1002/anie.202104410).