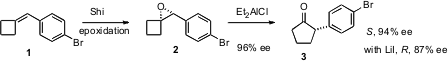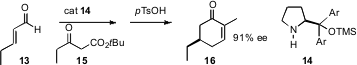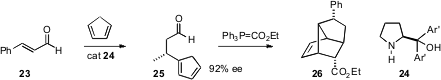A variety of effective strategies for catalytic enantioselective ring
construction have recently been developed. The simplest approach is to start
with a prochiral ring. Yian Shi of Colorado State University has applied (Angew.
Chem. Int. Ed. 2006, 45, 1429.
DOI: 10.1002/anie.200501520)
his enantioselective
epoxidation protocol to arylidene cyclobutanes such as 1. PMID:24856309 The epoxide
2, formed in high ee, on
pinacol rearrangement gives the
cyclopentanone 3
with retention of the high ee. Alternatively, exposure of 2 to LiI gives
the opposite enantiomer of 3, still in high enantiomeric excess.
For the same approach with arylidene
cyclopropanes to prepare
cyclobutanones,
see J. 1416990-09-4 Chemical name Org. Chem. 13-Bromotridec-1-ene Chemscene 2006, 71, 9519.
DOI: 10.1021/jo061341j).
A variety of enantioselective
Diels-Alder catalysts work well with highly
reactive dienes and dienophiles. Hisashi Yamamoto of the University of Chicago
has developed (J. Am. Chem. Soc. 2006, 128, 9626.
DOI: 10.1021/ja062508t)
a chiral Bronsted acid 6 that promotes the cycloaddition of ethyl vinyl
ketone 5 to less reactive dienes such as 4 with high
enantioselectivity.
A variety of enantioselective Diels-Alder catalysts work well with highly
reactive dienes and dienophiles. Hisashi Yamamoto of the University of Chicago
has developed
(J. Am. Chem. Soc. 2006, 128, 9626.
DOI: 10.1021/ja062508t)
a chiral Bronsted acid 6 that promotes the cycloaddition of ethyl vinyl ketone
5 to less reactive dienes such as 4 with high enantioselectivity.
Two elegant methods for enantioselective organic catalyst-mediated Michael
addition leading to substituted
cyclohexanes in high enantiomeric purity have
appeared. The first, reported (Nature 2006, 441, 861.
DOI: 10.1038/nature04820)
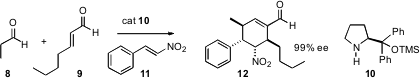
by Dieter Enders of RWTH Aachen, involves the condensation of an aldehyde, an
α,β-unsaturated aldehyde and an aryl nitroalkene, mediated by the now-common
proline-derived catalyst 9. A key question is whether alkyl nitroalkenes work as
well.
A complementary approach was reported (Chem. Commun. 2006, 4928.
DOI: 10.1039/b611366d)
by Karl
Anker Jørgensen of Aarhus University. Using the proline-derived catalyst 14 that
he has championed, Professor Jørgensen condensed α,β-unsaturated aldehydes such
as 13 with t-butyl acetoacetates such as 15, to give, after cyclization and
hydrolysis with p-TsOH, the 5-alkyl
cyclohexenone 16 in high ee.
Two elegant strategies for the enantioselective construction of bicyclic
systems have also appeared. Scott E. Schaus of Boston University, building on
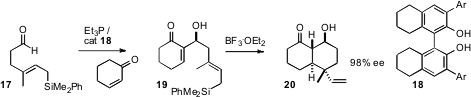
his earlier observation that the catalyst 18 directs the aldol condensation (Morita-Baylis-Hillman
reaction) of cyclohexenone to aldehydes with high ee, has applied (Angew. Chem.
Int. Ed. 2006, 45, 4929.
DOI: 10.1002/anie.200601076)
the reaction to a series of aldehydes such as
17 bearing unsaturated silanes. Exposure of the intial adducts 19 to BF3.OEt2 leads
to cyclization to the bicycle 20, in high ee.
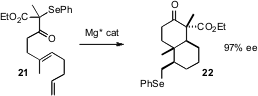
Dan Yang of the University of Hong Kong has been developing the chiral Lewis
acid-mediated radical cyclization of selenyl ketones such as 21. She has now
found (Angew. Chem. Int. Ed. 2006, 45, 255.
DOI: 10.1002/anie.200503056)
a Mg catalyst that directs both
monocyclic and bicyclic cyclization in high ee.
Yujiro Hayashi of the Tokyo University of Science has devised (Angew. Chem.
Int. Ed. 2006, 45, 6853.
DOI: 10.1002/anie.200602925)
a very short approach to tricyclic products such as
26. Using yet another proline-derived catalyst, cyclopentadiene added in conjugate
fashion to cinnamaldehyde 23 to give 25 as an inconsequential of double bond
isomers. Homologation followed by warming then gave the tricyclic adduct 26 in
high ee.