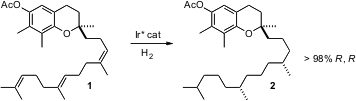
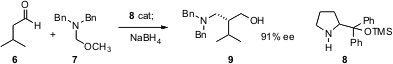One of the simplest ways to establish an enantiomerically-pure ternary center is to selectively hydrogenate a trisubstituted alkene. While there are many examples of the enantioselective reduction of more highly substituted alkenes, Andreas Pfaltz of the University of Basel is the first to report (Science2006, 311, 642. DOI: 10.1126/science.1121977)a family of catalysts that reduce simple alkyl-substituted alkenes with high ee. Formula of 112776-84-8 The efficacy of this approach was illustrated in spectacular fashion by the reduction of 1 to γ-tocopheryl acetate2. The authors demonstrated that the ring stereogenic center did not direct the hydrogenation. 152754-55-7 site
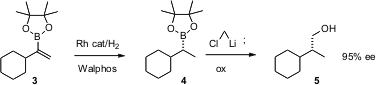
In a conceptually related approach, James P. Morken, now of Boston College, has found (Org. Lett. 2006, 8, 2413. PMID:23773119 DOI: 10.1021/ol060735u)that Rh/Walphos catalyses the reduction of vinyl boronates such as 3 to the saturated boronate 4 with high ee. The product 4 was homologated and oxidized to give 5 without loss of enantiomeric excess. Academic investigators should note that the Walphos ligands used in this study were donated by Solvias under the University Ligand Kit Program.
Samuel H. Gellman of the University of Wisconsin has shown (J. Am. Chem. Soc. 2006, 128, 6804. DOI: 10.1021/ja061731n)that the organocatalyst 8 mediates the Mannich condensation of 6 with 7 to give, after reduction, the amino alcohol 9 in high ee. The inclusion of LiCl is required to achieve the ee’s reported. The homologation works well for a range of aliphatic aldehydes.
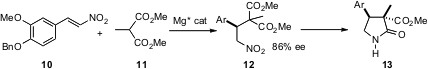
Enantioselective addition of malonate to nitro alkenes such as 10 has been extensively developed. Masahiro Terada of Tohuko University recently described (J. Am. Chem. Soc. 2006, 128, 1454.DOI: 10.1021/ja057848d)a new guanidine catalyst for this transformation. This article includes a thorough overview of the transition metal catalysts and of the organocatalysts that have been used for this transformation. Paul J. Nichols of Array BioPharma Process in Boulder, CO has found (Org. Lett. 2006, 8, 1495.DOI: 10.1021/ol060398p)that ee’s are maintained with alkyl malonates such as 11. The crystalline 12 was isolated in 87% yield in 87% ee on a 4 kg scale. Reduction and cyclization led to the lactam 13 in 4:1 dr, establishing a chiral quaternary center.
Tamio Hayashi of Kyoto University has developed (J. Am. Chem. Soc. 2006, 128, 5628. DOI: 10.1021/ja061430d)a general route to chiral quaternary centers, based on the Rh-catalyzed chiral conjugate of aryl boronic acids to imides such as 14. Remarkably, bond formation occurs selectively at the more substituted position of 14.
Organometallic catalysis allows the development of reactions that are beyond the range of conventional C-C bond formation. One such is illustrated by the hydrovinylation of styrene derivatives, including 17, with ethylene, developed (J. Am. Chem. Soc. 2006, 128, 5620.DOI: 10.1021/ja060999b)by T. V. RajanBabu of Ohio State University. This appears to be a general route to cyclic and acyclic chiral quaternary centers. An alternative protocol for the hydrovinylation of substituted styrenes was published earlier in the year (J. Am. Chem. Soc. 2006, 128, 2780.DOI: 10.1021/ja057654y)by Qi-Lin Zhou of Nankai University. The RajanBabu procedure appears to have advantages in efficacy and practicality, including both the commercial availability of the chiral ligand employed, and the ee’s achieved.