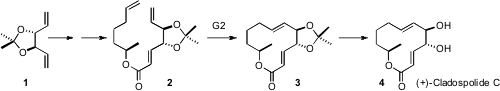
While alkene metathesis has become part of the toolkit of organic synthesis, investigators around the world are pushing the limits of the method as they employ the reaction in total synthesis. Duen-Ren Hou of National Central University, Taiwan prepared (J. Org. Chem. 2006, 71, 9887.DOI: 10.1021/jo061767y)the triene 2 from 1, itself easily prepared from D-mannitol. PMID:23865629 Metathesis proceeded cleanly, leading to (+)-cladospolide C. 3-Chloro-5-nitro-1H-pyrazole web
Yet, attempts at the alternative metathesis, closing the other alkene, gave no useful yield.
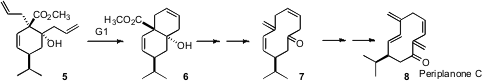
Ring-closing metathesis to form medium rings often leads to mixtures of Z and E alkenes. If the Z is desired, alkyne metathesis followed by selective hydrogenation can be employed. Radomir N. Saicic of the University of Belgrade has put forward (J. Org. Chem. 2006, 71, 9411.DOI: 10.1021/jo061790j)an alternative. 4-Ethynylpiperidine hydrochloride In stock RCM of 5 of course gave the Z alkene. Reduction followed by selective mesylation of the primary alcohol and Grob fragmentation then delivered the Z,Z-triene 7, which was readily carried on to the cockroach pheromone periplanone C (8).
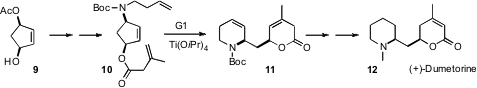
Ring-rearrangement metathesis is a powerful strategy for polycyclic construction. This is nicely illustrated by the synthesis of (+)-dumetorine recently reported (Tetrahedron Lett. 2006, 47, 7977.DOI: 10.1016/j.tetlet.2006.08.114)by Siegfried Blechert of the Technisches Universität, Berlin. There are at least three other precursors one can draw that could be precursors to 11. The cyclopentene 10 was chosen partly because it was readily available in enantiomerically-pure form, and also because the modest ring strain of the cyclopentene might drive the conversion of 10 to 11.
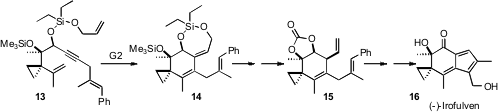
The semsynthetic illudin (-)-irofulven 16 is in clinical trials as an anti-cancer agent. Mohammad Movassaghi of MIT has described (Angew. Chem. Int. Ed. 2006, 45, 5859.DOI: 10.1002/anie.200602011)a concise route to the illudins. The key step was a boldly-conceivedenyne ring-closing metathesis (EYCRM) that converted 13 to 14. An additional ring-closing metathesis on 15 followed by oxidation then delivered 16.
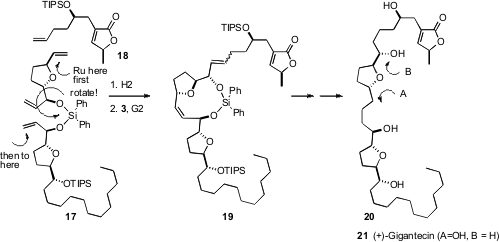
Although the usual relative rates of ring formation often guide metathesis, such is not always the case. In a recent synthesis of (+)-gigantecin (21) (Org. Lett. 2006, 8, 3383.DOI: 10.1021/ol061383u), Thomas R. Hoye of the University of Minnesota anticipated that 17 should first close the seven-membered ring, and then that product would participate in cross-metathesis with 18, leading to 21. The reaction, followed by mass spectrometry, seemed initially to be going well. In fact, however, 17 could freely rotate. The Ru catalyst engaged first with the most accessible alkene, then closed the eleven-membered ring, to give, after subsequent cross metathesis, the undesired 19, and so 20. The problem was solved, and the synthesis of the desired 21 accomplished, by first carrying out cross metathesis of 17 with an excess of 18, then effecting ring-closing metathesis to form the seven-membered ring.