The groups of Laurent El Kaïm and Laurence Grimaud from Ecole Nationale Supérieure de Techniques Avancées in Paris have reported on the generation of quinoxaline scaffolds (Heterocycles 2007, 73, ASAP. Link). The reaction sequence consists of an Ugi four-component reaction with subsequent Smiles rearrangement leading to intermediate 1. The Ugi-Smiles intermediate 1 further cyclizes to the quinoxaline product via an intramolecular Ullmann-type coupling. A higher yield of 62% could be obtained when the Ugi-Smiles intermediate is isolated prior to the Ullmann-type coupling step. PMID:23074147 By applying microwave heating for both steps the reaction time can be reduced from 31 h to only 1.8 h.

Tandem Bis-Aldol Reaction of Ketones
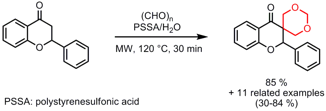
In order to attach a 1,3-dioxane moiety to ketones, Rajender Varma and Vivek Polshettiwar from the National Risk Management Research Laboratory, Ohio, have performed tandem bis-aldol reactions of ketones with paraformaldehyde (J. Org. Buy448-61-3 1-(2-Aminoethyl)piperidin-4-ol supplier Chem. 2007, 72, 7420. DOI: 10.1021/jo701337j). This one-pot protocol can be considered as eco-friendly, since polymer-supported PSSA and water as solvent are employed. Furthermore, phase separation under hot conditions occurred for most of the examples, thus facilitating the purification step (only decantation is needed).
Synthesis of Highly Sulfated Organic Scaffolds
Umesh Desai and coworkers from Virginia Commonwealth University have disclosed the sulfation of poly-hydroxyl scaffolds (Tetrahedron Lett. 2007, 48, 6754. DOI: 10.1016/j.tetlet.2007.07.100). This otherwise troublesome reaction proceeded well under microwave heating employing either Me3N∙SO3 (6-9 equiv/OH) and Et3N (10 equiv/OH) as base or SO3∙py (6-12 equiv/OH) and pyridine (10 equiv/OH), respectively, giving the per-sulfated products in high yields and short reaction times. With this method a range of functional groups are tolerated (amides, esters, aldehydes), alcoholic and phenolic OH-groups are sulfated equally well and the products are obtained in high purities.

Tetrasubstituted Pyrrole Synthesis
Till Opatz and Ines Bergner from the University of Mainz have synthesized 2,3,4,5-tetrasubstituted pyrroles by the reaction of α-(alkylideneamino)nitriles1 and α,β-unsaturated nitro compounds 2 under basic conditions (J. Org. Chem. 2007, 72, 7083. DOI: 10.1021/jo070426x). The reaction proceeds via cycloaddition of 1 and 2 with subsequent elimination of HCN and HNO2. Complete regioselectivity was achieved when Cs2CO3 was employed as base. The only exception being 4-cyanophenyl-substituted 2 (R3 = 4-CN-Ph); here isomeric mixtures of pyrroles were obtained. When microwave heating is applied, the reaction time could be reduced from several hours in refluxing THF to only 2 min at 100 °C.
