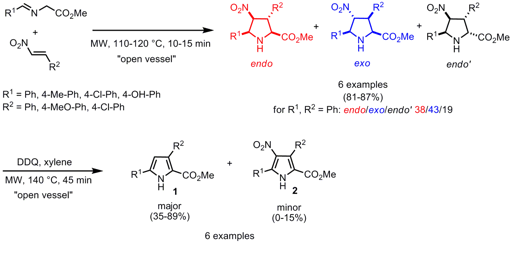
The groups of Fernando Cossio from Universidad del País Vasco-Euskal Herriko Unibertsitatea and Angel Díaz-Ortiz from Universidad de Castilla-La Mancha, Spain, have made investigations toward the stereochemical outcome for the [3+2] cycloaddition of stabilized azomethine ylides and nitrostyrenes (J. Org. Chem. 2007, 72, 4313.DOI: 10.1021/jo062672z). 5-Iodobenzo[b]thiophene In stock By performing the reactions without any solvent, three stereoisomers (endo, exo and endo´) of the pyrrolidine products are obtained, regardless if the cycloaddition is conducted under microwave or conventional heating. 625120-14-1 Chemical name Further aromatization of the corresponding pyrrolidines withDDQ in refluxing xylene, again under microwave irradiation, gives pyrrole-2-carboxylate 1 as major and 2 as minor product.
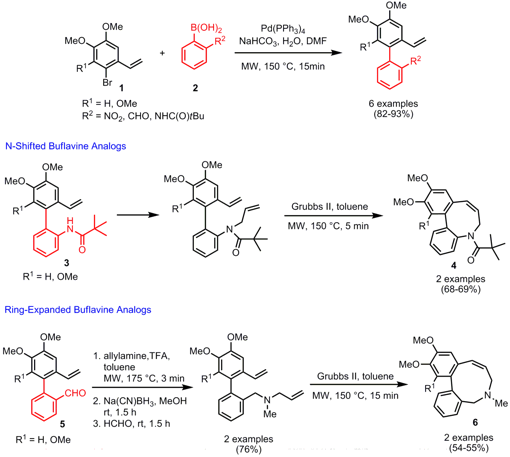
Synthesis of Buflavine Analogs
Erik Van der Eycken and co-workers from the University of Leuven have reported on the synthesis of N-shifted and ring-expanded buflavine analogs (Chem. Eur. J. PMID:23983589 2007, 13, ASAP.DOI: 10.1002/chem.200700177). Key steps in both reaction pathways are the Suzuki coupling of highly electron rich aryl halides 1 with ortho-substituted boronic acids 2 followed byring closing metathesis (RCM) for the medium-sized ring construction. The N-shifted buflavine derivatives 4, possessing an eight-membered ring, were obtained by allylation of biaryls 3 and subsequent RCM utilizing Grubbs second-generation catalyst. For the preparation of the nine-membered ring buflavine scaffolds 6, reductive amination was first performed on the biaryl aldehydes 5 followed by RCM. The double bond in both derivatives 4 and 6 was reduced by Pd-catalyzed hydrogenation to obtain the corresponding buflavine analogs.
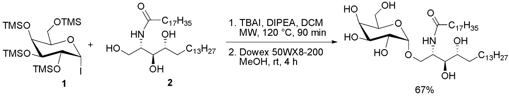
Synthesis of α-Linked Glycolipids
The group of Jacquelyn Gervay-Hague from the University of California Davis has discovered that microwave heating can accelerate the α-glycosidation of per-O-silylated galactosyl iodide 1 with ceramide 2 (Chem. Comm. 2007, 2336. DOI: 10.1039/b702551c). If galactosyl iodide 1 is reacted with ceramide 2 under conventional heating or at room temperature for 48 h, low yields are obtained. The authors assume that the unsaturated side chain in 2 favors lipid packing which could be overcome by applying microwave heating, thus obtaining the glycolipid product in higher yield. With this method which normally gives high yields when the reaction is performed at rt, only the α-isomer is obtained, unsaturated fatty acid side chains are tolerated and multiple protection-deprotection steps are eliminated.
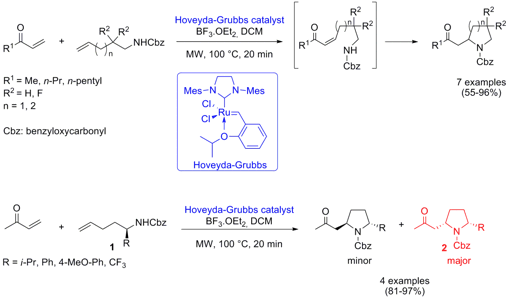
Tandem Cross Metathesis-Intramolecular Aza-Michael Reaction
Cyclic β-amino carbonyl derivatives were synthesized via a cross metathesis-aza-Michael tandem process by Santos Fustero and co-workers from Universidad de Valencia (J. Am. Chem. Soc. 2007, 129, 6700.DOI: 10.1021/ja0709829). Vinyl ketones are reacted with Cbz-protected amines to give pyrrolidines (n = 1) and piperidines (n = 2), respectively, in good to excellent yields. Key to the success of the reaction sequence was the combination of Hoveyda-Grubbs catalyst and BF3.OEt2 as Lewis acid that activates the cyclization step. By applying microwave heating the reaction time could be dramatically reduced from 4 days (45 °C) to only 20 min. Interestingly, under microwave heating an inversion of the selectivity was observed when α-substituted amines 1 were used, giving pyrrolidine derivatives 2 as major diastereoisomers.