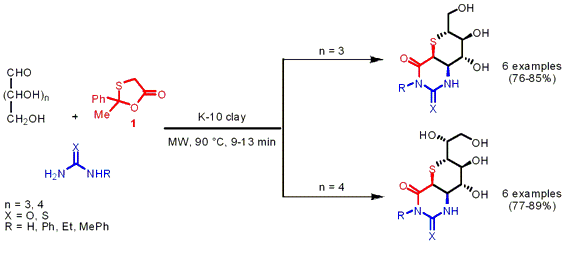
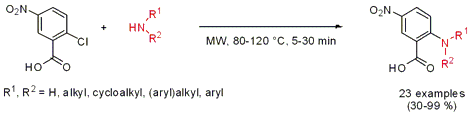The group of Lal Dhar Yadav from the University of Allahabad, India, has reported on a Biginelli-type reaction of unprotected aldoses, 2-methyl-2-phenyl-1,3-oxathiolan-5-one 1 as CH-acidic building block and urea/thiourea under acidic Montmorillonite K-10 catalysis and solvent-free conditions (Tetrahedron Lett. PMID:23935843 1500974-00-4 Chemscene 2007, 48, 4899.DOI: 10.1016/j.tetlet.2007.05.057). By employing the unprotected aldoses D-xylose (n = 3) and D-glucose (n = 4) a one-pot protocol was feasible avoiding protection and deprotection steps, furthermore they induce asymmetry to the final products. Thiosugar-annulated dihydropyrimidines were obtained via intermolecular domino cyclocondensations with >95% diastereoselectivity in favor of the cis isomer. 470482-44-1 Chemscene
Palladium-Catalyzed Spiro-Cyclizations
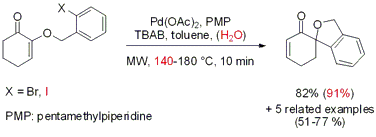
The Larhed group from Uppsala University has disclosed the regioselective synthesis of spiro[cyclohexane-1,1’-isobenzofuran] derivatives via intramolecular Heck cyclization (J. Org. Chem. 2007, 72, 5851. DOI: 10.1021/jo0708487). Five-exo cyclization of o-bromobenzyl cyclohexenyl ethers proceeded at 180 °C within 10 min. When o-iodobenzyl cyclohexenyl ethers are employed as substrates the addition of 5% of water (v/v) to the solvent system proved to promote the cyclization within 10 min at 140°C. Conventional heating at 100°C provided the products in similar yields but a reaction time of 18 h is required.
Synthesis of β-Amino Aldehydes
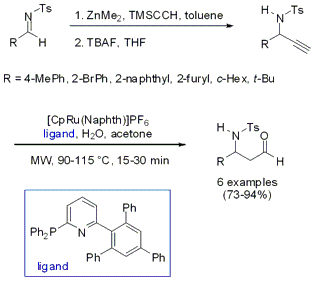
The synthesis of N-protected β-amino aldehydes via an alkynylation/anti-Markovnikov alkyne hydration sequence was developed by the groups of Lukas Hintermann and Carsten Bolm from RWTH Aachen University (J. Org. Chem. 2007, 72, 5704. DOI: 10.1021/jo070745o). By performing the Ru-catalyzed hydration under microwave heating the reaction times could be reduced from several hours at 55 °C to a maximum of 30 min. Additionally, for most of the derivatives the catalyst loading could be reduced to 5 mol%, compared to thermal heating, without any decrease in yields.
Catalyst-Free Aminations
The synthesis of N-substituted 5-nitroanthranilic acid derivatives was disclosed by Christa Müller and Younis Baqi from the University of Bonn (J. Org. Chem. 2007, 72, 5908.DOI: 10.1021/jo070731i). The amination of 2-chloro-5-nitrobenzoic acid proceeded smoothly with a variety of aryl- and alkylamines under catalyst- and solvent-free microwave conditions. Aliphatic primary and secondary amines only needed 5 min to give the products in quantitative yields. In the absence of the NO2-group in para-position or the carboxylate in ortho-position, respectively, no reaction occurred even under harsh reaction conditions (200°C, 3h).