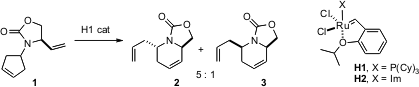
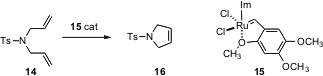Diastereoselectivity in ring-closing metathesis can be achieved under either kinetic or thermodynamic control. PMID:23539298 Siegfried Blechert of the Technische Universität Berlin has found (Angew. Chem. Int. Ed. 2006, 45, 1302. DOI: 10.1002/anie.200503321)that using the first-generation Hoveyda catalyst, 1 cyclizes to 2 and 3 with useful diastereocontrol. Product 2 was recovered unchanged on resubmission to the reaction conditions, indicating that in this case, the ring-closing metathesis is under kinetic control. There are many other examples in this paper, that is worth reading in detail.
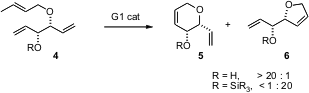
Bernd Schmidt of the Universität Potsdam has observed (Chem. Commun. 3-Bromo-5-methylpyrazin-2(1H)-one uses 2006, 2489. DOI: 10.1039/b604359c)high chemoselectivity in the ring-closing metathesis of 4. For protected 4, the reaction proceeded to give the expectedcyclopentene 6. The free alcohol, however, cyclized to 5, suggesting a directing or activating influence of the free OH. Cyclic ethers such as 5 and 6 can easily be oxidized to the correspondinglactones. Price of 1,2-Dideoxy-D-ribofuranose
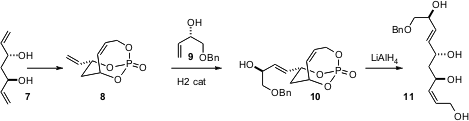
Paul R. Hanson of the University of Kansas has developed (Org. Lett. 2006, 8, 1673.DOI: 10.1021/ol0602809)a creative program around phosphates such as 8 derived from the diol 7. Cross metathesis with the allylic alcohol 8, for instance, proceeds without interference from the cyclic alkene. Reductive removal of the protecting phosphate then delivered the triol 11. The cyclic phosphates are also substrates for diastereoselective cuprate coupling.
Although the first and second generation Grubbs and Hoveyda catalysts are very good, there is still room for the development of new catalytic complexes. Ricardo Castarlenas and Pierre H. Dixneuf of the Université de Rennes have described (J. Am. Chem. Soc. 2006, 128, 4079.DOI: 10.1021/ja0579762)indylidene Ru complexes that are very active catalysts for alkene metathesis. Robert H. Grubbs of Caltech has devised (J. Am. Chem. Soc. 2006,128, 3508. DOI: 10.1021/ja058451c)a Ru complex G2 (aq) that is stable and active in water. Inter alia, this allows the ready equilibration, in water, of the inexpensive cis diol 12 to the trans diol 13, a valuable starting material for target-directed synthesis.
Karol Grela of the Polish Academy of Sciences in Warsaw has prepared (Chem. Commun. 2006, 841.DOI: 10.1039/b517088e)the dimethoxy Hoveyda Ru complex 15. In what promises to be a very useful protocol, after the cyclization of 14 to 16 in CH2Cl2 is complete, the reaction mixture is simply filtered through a pad of silica gel with additional CH2Cl2. This delivers the pure product 16, containing only 110 ppm Ru. Elution of the silica pad with EtOAc then brings the recovered catalyst 15, that can be re-used.
Alkyne metathesis is usually carried out with W and Mo carbyne complexes. Jeffrey S. Moore of the University of Illinois has developed (Angew. Chem. Int. Ed. 2006, 45, 585.DOI: 10.1002/anie.200502840)a protocol for depositing a Mo carbyne catalyst on silica gel. The resulting supported catalyst is very active at room temperature in toluene. Remi Chauvin of the Laboratoire de Chimie de Coordination in Toulouse has prepared (Tetrahedron Lett. 2006, 47, 2155.DOI: 10.1016/j.tetlet.2006.01.120)a less active but even more convenient catalyst by combining Mo(CO)6,p-chlorophenol, and the alkyne, then heating at 85°C in 1,2-dichloroethane. Both aromatic and aliphatic alkynes are smoothly dimerized.