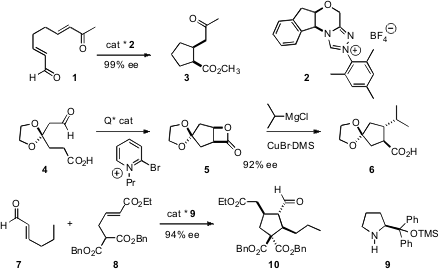
With the development of more advanced applications fororganocatalysts, several new approaches have been developed for the enantioselective construction of cyclopentanes. Karl A. Scheidt of Northwestern University has found (Angew. 2908-71-6 supplier Chem. 4-Bromo-1-(3-fluorophenyl)-1H-pyrazole Purity Int. Ed. 2007, 46, 3107. DOI: 10.1002/anie.200605235)that the enolates derived from addition of the catalyst 2 to the unsaturated aldehyde 1 show good facial selectivity in the ensuing intramolecular Michael addition, leading to 3 in high ee. PMID:24318587 Daniel Romo of Texas A&M had already developed the organocatalyst-mediated construction of cyclic β-lactones such as 5. Now, he has shown (Org. Lett. 2007, 9, 2111. DOI: 10.1021/ol070572p)that under Cu catalysis, 5 will couple withGrignard reagents to give the substituted cyclopentane 6 with clean inversion. Wei Wang of the University of New Mexico and Yun Tang of East China University of Science & Technology, Shanghai, using the simple catalyst 9, have devised (Angew. Chem. Int. Ed. 2007, 46, 3732.DOI: 10.1002/anie.200700485)a cascade process, combining7 and 8 to give the cyclopentane 10.
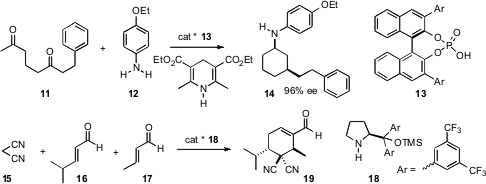
Cyclohexanes have also been prepared using organocatalysts. Benjamin List of the Max-Planck-Institut, Mülheim, has developed (J. Am. Chem. Soc. 2007, 129, 7498. DOI: 10.1021/ja072134j)a tandem aldol condensation – conjugate reduction – reductive amination procedure that converted the diketone 11 into the equatorial amine 14. Note that in the absence of the organocatalyst, the intramolecularaldol condensation of 11 would be expected to give the alternative regioisomer of the intermediate cyclohexenone. In a related development, Karl Anker Jørgensen of Aarhus University has established (Angew. Chem. Int. Ed. 2007, 46, 1101.DOI: 10.1002/anie.200604479)that the18-mediated Michael addition of 15 first to 16, then to 17, not only proceeded with high ee, but that the subsequent aldol condensation was also highly regioselective, leading to 19.
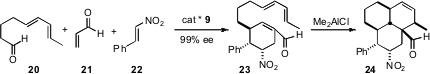
The cycloalkanes assembled using these strategies can then serve as directing scafffolds for further ring construction. Dieter Enders of RWTH Aachen has established (Angew. Chem. Int. Ed. 2007, 46, 467.DOI: 10.1002/anie.200603434)that23, prepared by 9-mediated Michael addition and aldol condensation of 20, 21, and 22, cyclized to 24 with high diastereocontrol.
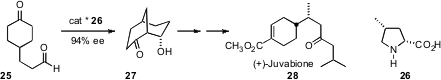
Organocatalysis can also be used to establish challenging side chain stereocenters. Yoshiharu Iwabuchi of Tohoku University used (Chem. Comm. 2007, 1175. DOI: 10.1039/b616641e)the bicyclic framework of 27 to guide conjugate addition, leading to the juvenile hormone (+)-juvabione (28) with high enantio- and diastereocontrol.
Note that most if not all of the catalysts mentioned here are commercially available, making the chemistry described easily accessible.