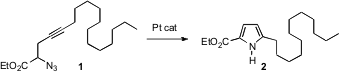
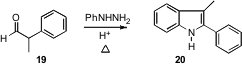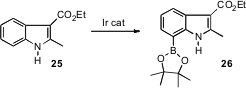Kou Hiroya of Tohoku University has described (Org. Lett. 2006,8, 5349. PMID:31085260 DOI: 10.1021/ol062249c)a versatile new route to pyrroles 2, based on the Pt-catalyzed rearrangement of azido alkynes such as 1. A wide range of functional groups appear to be tolerated.
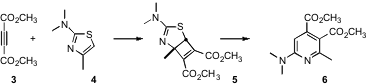
Mateo Alajarín of the Universidad de Murcia has reported (J. Org. Chem. 2006, 71, 5328. 2,2-Dimethyl-morpholine Price DOI: 10.1021/jo060664c)a new route to substituted pyridines 6, based on the the addition of dimethylacetylene dicarboxylate (3) to 2-aminothiazoles such as 4. The reaction is apparently proceeding by way of the [2+2] adduct 5. 3-Butynoic acid Price
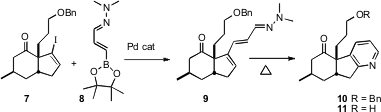
In a synthesis of (+)-lycopladine A (11), F. Dean Toste of the University of California, Berkeley demonstrated (Angew. Chem. Int. Ed. 2006, 45, 5991. DOI: 10.1002/anie.200602035)the power of pyridine construction. Coupling of7 with the hydrazone 8 gave 9, which on heating smoothly converted to 10.
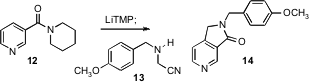
Substituted pyridines can also be prepared from preformed pyridines. Laurent Bischoff of IRCOF-INSA, Rouen, taking advantage (Org. Lett. 2006,8, 5889. DOI: 10.1021/ol062577c)of the known preference for 4-metalation of nicotinamides such as 12, condensed the resulting anion with the Mannich equivalent 13 to give, after cyclization, the lactam 14.
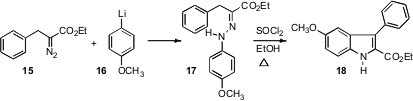
The classic route to indoles is the Fischer synthesis, acid-mediated cyclization of an aryl hydrazone such as 17. Norio Takamura of Musashino University, Tokyo has reported (Tetrahedron Lett. 2006, 47, 743. DOI: 10.1016/j.tetlet.2005.11.126)that such hydrazones can be simply prepared by the addition of an aryl lithium to an α-diazo ester.
The Fischer synthesis works with aldehydes also. Remarkably, unsymmetrical α-branched aldehydes such as 19 had not been studied in detail. Kevin G. Liu of Wyeth Research in Princeton, N.J. has now found (Org. Lett. 2006, 8, 5769.DOI: 10.1021/ol0623567)that the cyclizations work well, and that the requisite 1,2-shift en route to 20 proceeds with high selectivity.
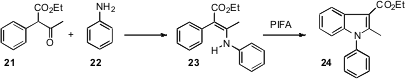
A complementary approach to indoles begins with an alkyl substituent on the benzene ring. Kang Zhao of Tianjin University has devised (Org. Lett. 2006, 8, 5919. DOI: 10.1021/ol062288o)conditions for the oxidative cyclization of an enamine such as 23 to the indole24.
An alternative is to directly functionalize the indole nucleus. Robert E. Malezcka, Jr. and Milton R. Smith III of Michigan State University have shown (J. Am. Chem. Soc. 2006, 128, 15552.DOI: 10.1021/ja0631652)that using an Ir catalyst, it is possible to effectively borylate the 7-position of a wide variety of indoles.