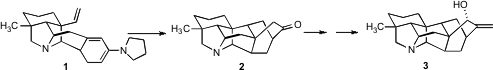
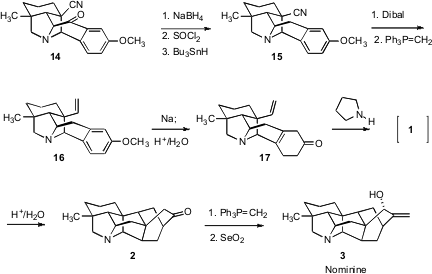
Retrosynthetic analysis of the heptacyclic ring structure of nominine (3), a representative member of the hetisine family of alkaloids, is not for the faint of heart. David Y. 334905-81-6 manufacturer Gin, now at the Sloan-Kettering Institute, has presented (J. Am. PMID:23577779 Chem. Soc. Buy2-(Difluoromethyl)pyridin-4-amine 2006, 128, 8734. DOI: 10.1021/ja0625430)an elegant solution to this problem. One key step in the synthesis was the intramolecular Diels-Alder cyclization of 1 to 2.
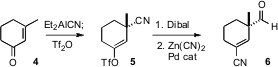
The convergent construction of 1 by dipolar cycloaddition presented some interesting challenges. One half was prepared from 3-methylcyclohexenone (4). Addition of Et2AlCN followed by triflation gave 5 in regiocontrolled fashion. Reduction followed by Pd-catalyzed coupling of the enol triflate then led to the aldehyde 6. An intriguing question is how one would prepare 6 in enantiomerically pure form.
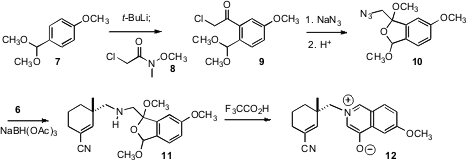
The other half of 1 was prepared from the acetal 7. Lateral metalation followed by acylation with the Weinreb amide 8 gave the ketone9. Displacement with azide followed by exposure to acid gave the bridged acetal 10, which was condensed reductively with 6 to give the oxidopyridinium betaine 12.
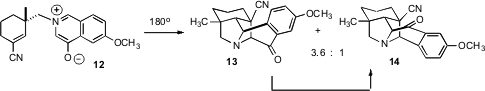
Two products, 13 and 14, could arise from the dipolar cycloaddition of 12. It was not clear at the outset which would be preferred. In the event, it did not matter. Even though the thermal equilbrium favored the undesired 13, equilibration was efficient, and the two were easily separated.
With 14 in hand, the stage was set for the intramolecular Diels-Alder cyclization. In fact, the diene 1 was not isolated. The evidence for the intermediacy of 1 was the observation that on exposure to pyrollidine in methanol at 60°C, the Birch reduction product 17 smoothly cyclized to 2. Methylenation followed by kinetic, axial-selectiveSeO2 oxidation then completed the synthesis of 3.
The concise elegance of this 17-step synthesis of the heptacyclic hetesine alkaloid nominine 3 is all the more apparent when it is compared with the only previously-reported synthesis, published just two years earlier, which took 40 steps.