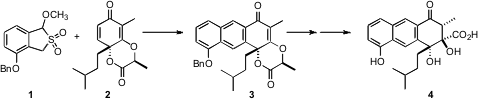
(+)-Rishirilide (4), an inhibitor of α2-macroglobulin, a
tetrameric serum glycoprotein that is an irreversible protease inhibitor, has a
deceptively simple structure. Thomas R. R. Pettus of the University of
California at Santa Barbara has reported (J. Am. Chem. 351439-07-1 Formula Soc. 2006,
128, 15625.
DOI: 10.1021/ja062987w
a concise route to 4, based on the highly regioselective Diels-Alder addition of
the enantiomerically-pure dienophile 2 to the diene derived from 1. Buy2-Octyldecanoic acid
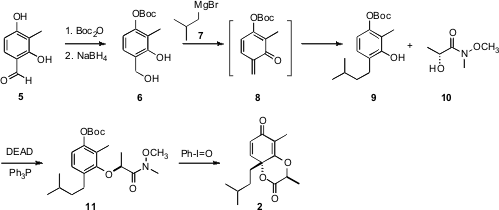
The preparation of 2 was carried out from the aldehyde 5. PMID:23891445 Selective
protection of the less hindered phenol followed by reduction gave the benzyl
alcohol 6. On exposure to excess Grignard reagent 7, the primary alcohol of
6 apparently underwent elimination to give the o-quinone methide
8, conjugate
addition to which gave 9.
Mitsunobu coupling of 9 with the lactic acid amide
10 proceeded with clean inversion, to give 11. The methoxy methyl amide of
11 was required for the oxidative dearomatization to 2 to proceed efficiently.
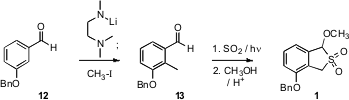
The preparation of the masked diene 1 started with the aldehyde 12. Following
the Comins procedure, metalation of the derived hemiaminal alkoxide and
subsequent addition of methyl iodide gave 13. Addition of SO2 to the photoenol
derived from 13 followed by etherification of the labile secondary alcohol so
formed then gave 1.
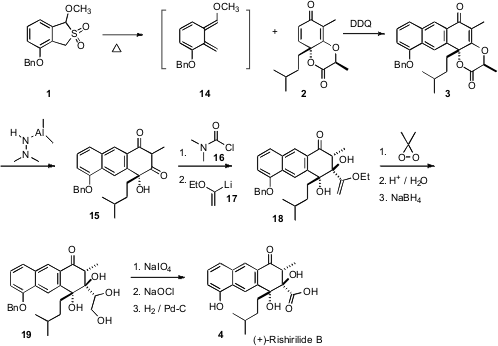
The dienophile 2 should undergo
cycloaddition with high facial selectivity.
That is not relevant in this application, as addition to the intermediate
quinone methide 14 was followed by elimination and oxidative aromatization. The
regioselectivity of the addition, delivering 2, was, however, critical for the
success of the synthesis.
With 3 in hand, what remained was a one-carbon homologation. This was
accomplished by selective O-acylation with dimethyl carbamoyl chloride 16.
Subsequent addition of an excess of anion 17 led, via alkoxide-directed addition
to the remaining ketone carbonyl followed by deacylation, to the desired adduct
18. Careful cleavage, to avoid lactone formation, then delivered 4. Note that
this synthesis of (+)-rishirilide B (4) would be classified as enantioselective,
since the intial stereogenic center of 2, that set the absolute configuration of
the dearomatized ring of 2, was not included in the final product.