The total synthesis of steroids such as (+)-digitoxigenin (3) has been studied for more than sixty years, yet it has never been thought that such studies would lead to a preparative route that would be competitive with partial synthesis from the abundant plant sterols. Price of HO-PEG24-OH The enantioselective synthesis of 3 recently (Tetrahedron Lett. 6-Bromo-3-hydroxypicolinic acid uses 2007, 48, 1541. DOI: 10.1016/j.tetlet.2007.01.024)described by Masahisa Nakada of Waseda University suggests that the preparative synthesis of even such complex polycarbocycles may in fact be practical. A key step in the synthesis of 3 was the conjugate addition of the cuprate derived from 1 to the enone 2. PMID:23715856
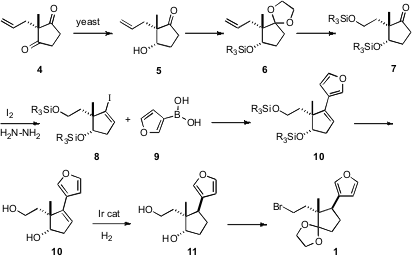
For this convergent approach to be effective, both 1 and 2 had to first be prepared in enantiomerically-pure form. The synthesis of 1 started with the bakers’ yeast reduction of the prochiral diketone 4 to give 5 in high ee. The key problem was the establishment of the ternary center of 1. This was accomplished by coupling the iodide 8 with the boronic acid 9. Hydroxyl-directed hydrogenation of 10 then led to the desired 1.
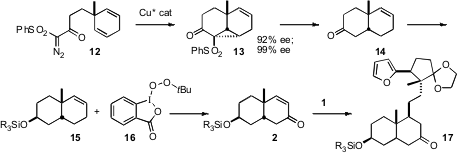
The preparation of 2 began with Cu-catalyzed cyclization of the prochiral diene 12 to give the crystalline 13. Recrystallization raised the ee of 13 to > 99%. Reductive opening of 13 gave 14, which was reduced and protected to give 15. Oxidation of 15 with the Ochiai reagent 16 provided the enone 2. Conjugate addition of1 proceeded across the more open face of the enone 2, to give 17.
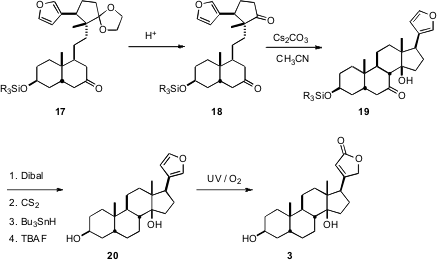
The key step to close the B ring of the steroid was the intramolecular aldol condensation of 18 to give 19. The β-hydroxy ketone so produced was too sensitive to reduce by direct methods, so the monoxanthate was prepared from the derived diol, and then reduced under free radical conditions, leading to the diol 20. Singlet oxygenation of 20 then completed the synthesis of 3.
Clearly, the strength of this synthesis is the elegant construction of the AB enone 2. Even more important is the demonstration of a general convergent route to the steroids, by addition of an enantiomerically-pure D ring moiety to an AB fragment. The power of asymmetric synthesis makes the precursors 1 and 2 readily available in the necessary enantiomerically-pure form.