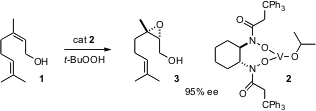
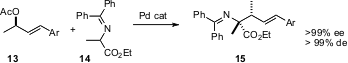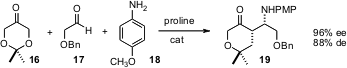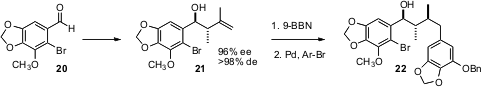Sharpless asymmetric epoxidation has long been a workhorse of enantioselective synthesis. The SAE protocol, however, gives only mediocre ee’s with cis allylic alcohols such as nerol (1). PMID:23381626 Hisashi Yamamoto of the University of Chicago has devised an alternative procedure (Angew. Chem. Int. Ed. 2005, 44, 4389. DOI: 10.1002/anie.200500938), based on the bis N-hydroxyamide 2. This gives high ee’s with a variety of substituted allylic alcohols, including1.
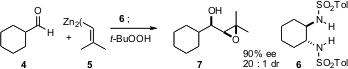
In a related development (J. Am. Formula of 1,18-Dibromooctadecane Chem. Soc. 2005, 127, 14668. DOI: 10.1021/ja051291k), Patrick J. Walsh of the University of Pennsylvania has added alkenyl zincs such as 5 to aldehydes such as 4 in the presence of the chiral ligand 6. Oxygenation in situ of the resulting zinc alkoxide delivers the epoxy alcohol 7 in high ee. The alkenyl zincs are prepared from the corresponding alkenyl bromides with Li and ZnBr2.
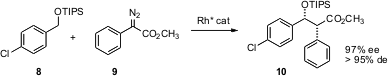
Arrays of stereogenic centers are often assembled by aldol condensation. Huw M.L. Davies of the University at Buffalo has put forward (J. Org. Chem. 2005, 70, 10737. 1172057-73-6 uses DOI: 10.1021/jo051747g)an alternative. Enantioselective Rh-mediated insertion of the carbene derived from 9 into the benzylic C-H of 8 gives the “protected aldol” product 10 in high ee.
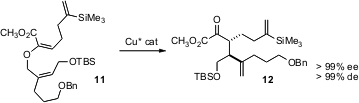
The Claisen rearrangement is a powerful method for assembling adjacent alkylated stereogenic centers. Martin Hiersemann of the Technische Universität Dresden has shown (Org. Lett. 2005, 7, 5705. DOI: 10.1021/ol052462t)that a chiral Cu catalyst mediates the conversion of 11 to 12. The α-keto ester12 so prepared was a single enantiomer of a single diasteromer. A limitation on this approach is the necessity of preparing the stereodefined enol ether starting material. Joseph M. Ready of UT Southwestern Medical Center has reported (Org. Lett. 2005, 7, 5681.DOI: 10.1021/ol052413g)an effective procedure for preparing such ethers.
Enantiomerically pure allylic acetates such as 13 are easily prepared. Motoi Kawatsura of Tottori University has devised conditions (Tetrahedron Lett. 2005, 46, 6663.DOI: 10.1016/j.tetlet.2005.07.139)for coupling13 with 14 with very high regio- and diastereocontrol.
Aldol reactions of 16 have been extensively reported. Bernhard Westermann of the Institute of Plant Biochemistry in Halle (Angew. Chem. Int. Ed. 2005, 44, 4077. DOI: 10.1002/anie.200500297)and Dieter Enders of RWTH Aachen (Angew. Chem. Int. Ed. 2005, 44, 4079.DOI: 10.1002/anie.200500810)have now independently shown that the Mannich condensation can also be successful. James C. Anderson of the University of Nottingham (J. Org. Chem. 2005, 70, 5665.DOI: 10.1021/jo050762i), Ming-Hua Xu and Guo-Qiang Lin of the Shanghai Institute of Organic Chemistry (J. Am. Chem. Soc. 2005, 127, 11956.DOI: 10.1021/ja054401w)and Scott E. Schaus of Boston University (J. Am. Chem. Soc. 2005, 127, 11256.DOI: 10.1021/ja0537373)have also reported significant progress with catalytic enantioselective Mannich reactions.
Strategies for the assembly of more extended arrays of stereogenic centers are also important. Robert S. Coleman of The Ohio State University found (J. Org. Chem. 2005, 70, 8932.DOI: 10.1021/jo051525i)that the best results for the homologation of 20 were achieved using the protocol developed by James L. Leighton of Columbia University. Diastereoselectivehydroboration then led to 22. In another development, William R. Roush of Scripps Florida has reported (Org. Lett. 2005, 7, 3941. DOI: 10.1021/ol0514303)further applications of his linchpin strategy for the assembly of arrays of stereogenic centers.