Last week ( 2006 Jan 23), aldol andMannich-based strategies for the catalytic enantioselective construction of extended arrays of aminated, oxygenated and alkylated stereogenic centers were reviewed. This week, the focus is on alternative strategies for the construction of extended arrays of stereogenic centers.
2006 Jan 23), aldol andMannich-based strategies for the catalytic enantioselective construction of extended arrays of aminated, oxygenated and alkylated stereogenic centers were reviewed. This week, the focus is on alternative strategies for the construction of extended arrays of stereogenic centers.
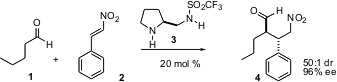
Organocatalysts have been used to effect transformations that might not have been expected to be practical. 88284-48-4 Formula Wei Wang of the University of New Mexico has reported (Angew. Chem. 5-Chloroquinolin-8-amine supplier Int. Ed. 2005, 44, 1369.DOI: 10.1002/anie.200461959)that the sulfonamide 3 mediates the enantioselectiveMichael addition of aldehydes to nitrostyrene derivatives, with impressive stereocontrol. PMID:25027343
Even free radical reactions can be carried out with high diastereoselectivity. Mukund P. Sibi of North Dakota State University has demonstrated (J. Am. Chem. Soc. 2005, 127, 2390.DOI: 10.1021/ja043371e)that reduction of an iodide 5 in the presence a catalytic amount of an enantiomerically-pure Lewis acidic Mg complex delivers the conjugate addition product 7 with remarkable control of both centers.
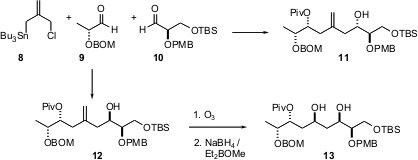
The stereocontrolled assembly of more extended arrays is also important. Gary E. Keck of the University of Utah has designed (J. Org. Chem. 2005,70, 2543. DOI: 10.1021/jo048308m)the linchpin chlorostannane 8. Sequential condensation of 8 with 9 and then with 10 could be directed toward either 11 (BF3.OEt2) or 12 (MgBr2). Ozonolysis of 12 followed by OH-directed reduction gave 13, a key intermediate toward the total synthesis of bryostatin.
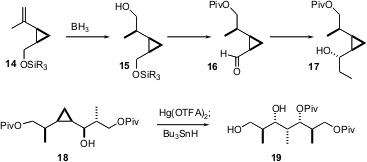
Janine Cossy of ESPCI, Paris has also developed (Tetrahedron 2005,61, 7632.DOI: 10.1016/j.tet.2005.05.099)an approach to extended arrays of stereogenic centers. Cis alkenyl cyclopropanes such as 14, readily available in high enantiomeric purity, undergo hydroboration with substantial diastereocontrol. Organometallic addition to the derived aldehyde 16 also proceeds with high diastereoselectivity. All of this is of interest because the hydroxy cyclopropanes are opened under oxymercuration conditions, to give, with clean pivaloyl transfer, the stereopentad 19. Although in this application the C-Hg was replaced by C-H, to yield a methyl group, there are many other ways to functionalize the intermediate organomercurial.
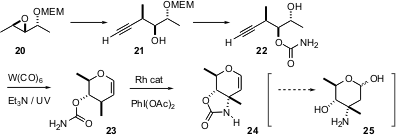
The recent (Org. Lett. 2005, 7, 1785.DOI: 10.1021/ol050356l)synthesis of D-saccharosamine (25) by Kathlyn A. Parker of SUNY Stony Brook is a showcase for the power of organometallic reagents in stereocontrolled organic synthesis. The starting material 20 was prepared bySharpless epoxidation. Acetylide opening was highly regioselective, to give 21. After protecting group exchange, tungsten-mediated cyclization of 22 gave23. Dubois cyclization of 23 proceeded selectively away from the ether methine, to give the protected D-saccharosamine 24.