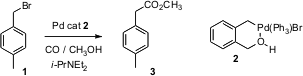
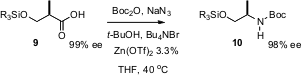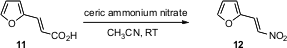W. Edward Lendsell and Peter N. Preston of Herriot-Watt University, Edinburgh, have developed (Tetrahedron Lett. 2005, 46, 8695. DOI: 10.1016/j.tetlet.2005.10.038)a new family of Pd catalysts, exemplified by the complex 2. PMID:24580853 These catalysts efficiently (1 mol %) mediate homologation of benzylic halides such as 1 to the corresponding methyl esters at low temperature (35°C) and modest CO overpressure (3.45 bar).
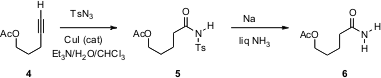
Sukbok Chang of KAIST, Daejon, Korea has uncovered (J. Am. Chem. Soc. 2005, 127, 16046. DOI: 10.1021/ja056399e)a truly remarkable reaction, the hydrative conversion of a terminal alkyne such as 4 to the N-sulfonyl amide5. The free amide 6 can be released by dissolving metal reduction. It is likely that 5 could be N-alkylated efficiently, including under Mitsunobu conditions. The sulfonyl amide 5 may also behave like a Weinreb amide, allowing efficient coupling with organometallic nucleophiles to form the corresponding ketones. 2166539-35-9 Price
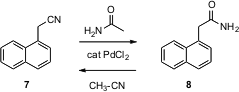
Another remarkable set of transformations has been reported (Org. Lett. 2005, 7, 5237. DOI: 10.1021/ol052100l)by Sonia I. Buy4-Chloro-1H-indole-7-carboxylic acid Maffioli of Vicuron Pharmaceuticals, Gerenzano, Italy. Exposure of a nitrile 7 to a catalytic amount (as little as 0.5 mol %) of PdCl2 (Pd(OAc)2 served equally well) in the presence of acetamide led to the primary amide 8. Conversely, the same catalyst in the presence of acetonitrile converts the primary amide 8 to the nitrile 7. These reactions run at or slightly above ambient temperature.
The one-carbon degradation of carboxylic acids is often used to install amines, converting the C-CO2H bond into a C-N bond. Hélène Lebel of the Université de Montréal has developed (Org. Lett. 2005, 7, 4107. DOI: 10.1021/ol051428b)a one-pot procedure leading directly to the N-Boc protected amine 10. Of the several catalysts investigated for this transformation, Zn(OTf)2 gave by far the best results.
J. Madhusudana Rao of the Indian Institute of Chemical Technology, Hyderabad, has developed (Tetrahedron Lett. 2005, 46, 8141. DOI: 10.1016/j.tetlet.2005.09.126)a simple protocol for the one-carbon degradation of an α,β-unsaturated carboxylic acid such as 11 to the corresponding nitro alkene 12. The other examples given were also β-aryl or β-heteroaryl substituted. It will be interesting to learn whether or not this transformation can be extended to aliphatic and alicyclic α,β-unsaturated carboxylic acids, as well as to α,β-unsaturated carboxylic acids such as 11 having additional substituents on the alkene.
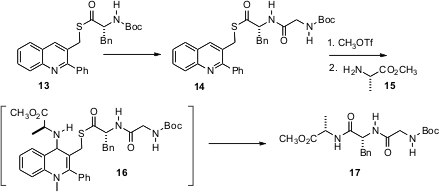
There have been several noteworthy advances in esterification (Kazuaki Ishihara, J. Am. Chem. Soc. 2005, 127, 4168. DOI), ester hydrolysis (K. C. Nicolaou, Angew. Chem. Int. Ed. 2005, 44, 1378.DOI: 10.1002/anie.200462207), and amide bond formation (Z. J. Kaminski, A. M. Papini, J. Am. Chem. Soc. 2005, 127, 16912.DOI: 10.1021/ja054260y). Of these, the most striking is the quinoline thio ester 13 developed (J. Am. Chem. Soc. 2005, 127, 15668.DOI: 10.1021/ja054775p)by Vincent Levacher of the Université de Rouen. The thioester is robust enough to stand up under normal Boc removal and peptide forming conditions, to give 14. N-Alkylation with Me triflate then activates the quinoline for addition by the free amine of 17, leading to intramolecular acylation and eventual release of the tripeptide 17. If such intramolecular acylation is effective with longer chain oligopeptides, this simple quinoline platform could enable the preparation of proteins by total synthesis.