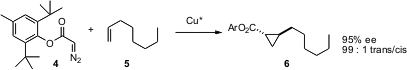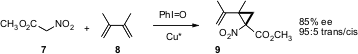Strained three- and four-membered rings can be used to constructlarger rings. The release of the strain energy facilitates bond formation, and the stereochemistry of the small ring can be transmitted to the larger ring.
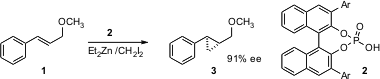
The first step in this approach is the stereocontrolled construction of the smaller ring. Significant progress in asymmetric cyclopropanation has been reported this year. André B. Charette of the Université de Montreal has found (J. Am. Chem. Soc. 2005, 127, 12440.DOI: 10.1021/ja0529687)that Binol-derived phosphoric acids directed the Simmons-Smith cyclopropanation of allylic ethers such as 1 with high ee. Allylic ethers react more rapidly than do isolated alkenes. Other alkyl and silyl ethers work well also. Patrick J. Walsh of the University of Pennsylvania has reported (J. Am. PMID:32695810 Chem. [Acr-Mes]+(ClO4)- web Soc. Buy1309377-79-4 2005, 127, 13138.DOI: 10.1021/ja0539239)a complementary approach that is equally effective for the high ee construction of cyclopropanes derived from allylic alcohols.
Preformed diazo carbonyl compounds such as 4 are common precursors to cyclopropanes. Andreas Pfaltz of the University of Basel has described (Angew. Chem. Int. Ed. 2005, 44, 4888.DOI: 10.1002/anie.200501111)a family of enantiomerically-pure Cu catalysts that effect alkene insertion. It is particularly noteworthy that these catalysts are effective even with simple alkyl-substituted alkenes such as 5.
Carbenes can also be generated directly from activated methylene compounds such as 7. Professor Charette (J. Am. Chem. Soc. 2005, 127, 18014. DOI: 10.1021/ja056192l)has been able to combine this approach with chiral Cu catalysis, leading to the cyclopropane amino acid precursor 9 in high de and ee.
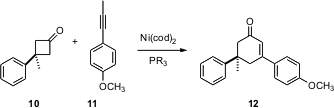
Release of ring strain from small rings can drive the formation of larger rings. Masahiro Murakami of Kyoto University has shown (J. Am. Chem. Soc. 2005, 127, 6932.DOI: 10.1021/ja050674f)that inexpensive Ni catalysts mediate the addition of an alkyne such as 10 to a cyclobutanone such as 11 to give, with substantial regiocontrol, the cyclohexenone 12. Michael E. Jung of UCLA has described (J. Am. Chem. Soc. 2005, 127, 11206.DOI: 10.1021/ja051663p)an alternative strategy for expanding readily-prepared cyclobutanols to cyclohexenones.
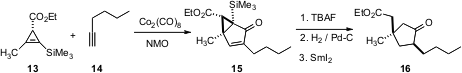
Cyclopropenes such as 13 are readily available in enantiomerically-pure form. Joseph M. Fox of the University of Delaware has shown (Org. Lett. 2005, 7, 3593.DOI: 10.1021/ol051456u)that unlike ordinary alkenes, these strained alkenes participate smoothly in Pauson-Khand cycloaddition, and that the additions proceed with very high regio- and diastereocontrol. The resulting cyclopropanes are readily carried on to substituted cyclopentanones such as 16.
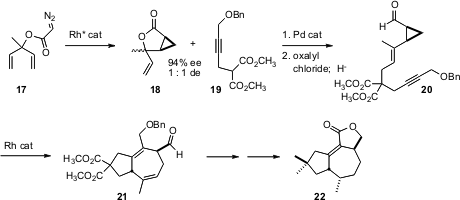
The utility of strained rings in target-directed synthesis is illustrated by the construction of tremulenolide A (22) recently reported (Org. Lett. 2005, 7, 4535.DOI: 10.1021/ol051945u)by Stephen F. Martin of the University of Texas. Cyclization of the prochiral diazo ester 17 with the Doyle MEPY catalyst delivered the cyclopropane 18 in high ee, as an inconsequential mixture of diastereomers. Pd-mediated coupling to 19 gave 20 with high geometric control. Rh-catalyzed opening, following the Wender precedent, gave 21, with the relative and absolute configuration derived from the cyclopropane of 20. The diester 21 was then carried on to 22.