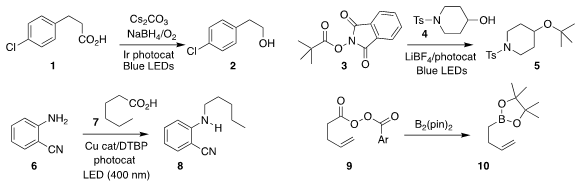
Zhankui Sun of Shanghai Jiao Tong University devised photolytic conditions
for the oxidation of the acid 1
to the alcohol 2
(J. Org. Chem. 2020, 85, 5019.
DOI: 10.1021/acs.joc.0c00312).
Kazunori Nagao and Hirohisa Ohmiya of Kanazawa University also
used photolysis to prepare the
tert-butyl ether 5 by coupling the
ester 3 with the alcohol 4
(J. Am. 4-bromopyrimidine hydrobromide site Chem. Soc. 2020, 142, 1211.
DOI: 10.1021/jacs.9b12335).
Oleg V. Larionov of the University of Texas at San Antionio
alkylated the amine 6 with the acid 7,
leading to the amine 8
(Angew. Chem. Int. Ed. 2020, 59, 7921.
DOI: 10.1002/anie.201916710).
Bing Han of Lanzhou University found that the peranhydride 9 could be converted directly to the
borate ester 10
(Org. Lett. 2020, 22, 234.
DOI: 10.1021/acs.orglett.9b04218). Methyl 4-bromo-1H-indole-7-carboxylate custom synthesis
Alexander O. Terentev of the N. D. Zelinsky Institute of Organic Chemistry
assembled 13 by coupling 11 with 12 under oxidative conditions
(J. Org. Chem. PMID:34856019 2020, 85, 1935.
DOI: 10.1021/acs.joc.9b02656).
Diwan S. Rawat of the University of Delhi demonstrated that triflic acid effectively
catalyzed the IBX oxidation of the alcohol 14
to the ketone 15
(Tetrahedron Lett. 2020, 61, 151749.
DOI: 10.1016/j.tetlet.2020.151749).
Matthias Beller of the Leibniz-Institute for Catalysis and Paul J. Dyson of the
École Polytechnique Fedérale de Lausanne devised a Co catalyst that directly
mediated the dehydrogenative cross-coupling of the amine 16 with
the amine 17, leading to the imine 18
(Angew. Chem. Int. Ed. 2020, 59, 7501.
DOI: 10.1002/anie.201915526).
Ohyun Kwon of UCLA effected the
oxidative cleavage of the alkene 19 to the ketone 20
(Angew. Chem. Int. Ed. 2020, 59, 1211.
DOI: 10.1002/anie.201913201).
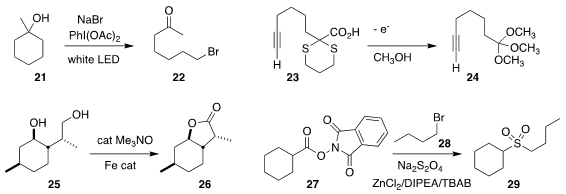
Jianbo Wang of Peking University oxidized the tertiary alcohol 21 to
the bromo ketone 22
(Chem. Commun. 2020, 56, 5002.
DOI: 10.1039/D0CC01720E). Zhong-Quan Liu of the
Nanjing University of Chinese Medicine reported related results
(ACS Catal. 2020, 10, 6603.
DOI: 10.1021/acscatal.0c01495).
Kevin Lam of the University of Greenwich devised a general
route to ortho esters such as 24, based on the electrochemical oxidation
of the corresponding
dithiane 23
(Org. Lett. 2020, 22, 4000.
DOI: 10.1021/acs.orglett.0c01324).
Timothy W. Funk of Gettysburg College demonstrated the selective oxidation of the diol
25 to the lactone 26
(J. Org. Chem. 2020, 85, 1823.
DOI: 10.1021/acs.joc.9b01884).
Ming Wang and Xuefeng Jiang of East China Normal University assembled the
sulfone 29 by the three-component coupling of the ester 27, the
bromide 28 and sodium dithionite
(Angew. Chem. Int. Ed. 2020, 59, 8907.
DOI: 10.1002/anie.202001589).
Under Ramberg-Bäcklund conditions, such a sulfone can readily
be converted to the corresponding alkene.
The new antibiotic baulamycin A (32) was isolated in low yield from
Streptomyces tempisquensis. In the course of a synthesis of 32, David
H. Sherman of the University of Michigan and (the late) Robert M. Williams of
Colorado State University used ozone on silica gel to convert the phenyl group
of 30 to the carboxylic acid of 31
(J. Org. Chem. 2020, 85, 3812.
DOI: 10.1021/acs.joc.9b03443).