N-heterocyclic salts are air-stable crystalline solids that can be stored
with no special precautions and are the precursors of N-Heterocyclic Carbenes (NHCs),
which are produced upon treatment with an appropriate base. The use of chiral
NHCs, especially those with an imidazolinylidene carbene scaffold, in asymmetric
organocatalytic transformations has received considerable attention in recent
years (Chem. Rev. 2000, 100, 39,
DOI: 10.1021/cr940472u;
Adv. Organomet. Chem. 2001, 46, 181,
DOI: 10.1016/S0065-3055(00)46004-1;
Angew. Chem. PMID:24268253 Int. Ed. 2002, 41, 1290,
DOI: 10.1002/1521-3773(20020415)41:8%3C1290::AID-ANIE1290%3E3.0.CO;2-Y;
Acc. Chem. Res. 2004, 37, 534,
DOI: 10.1021/ar030050j;
Chem Soc Rev. 2004, 33, 619,
DOI: 10.1039/b406802p). The preparation and utility of the related triazolium salts, which are unlike
their imidazolium salt counterparts, have also been explored. 2-Bromo-4-formylnicotinonitrile site This Highlight
presents the latest developments from 2005 to the present using the emergent NHC
chemistry.
1. Chiral NHCs
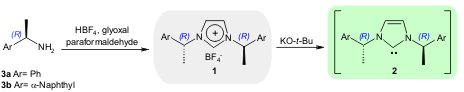
Maruoka and co-workers reported the synthesis of C2-symmetric
chiral imidazolium salts 1, which are the precursors of chiral NHC
catalysts 2, starting from readily available chiral amines 3 (Org.
Lett. 2005, 7, 1347.
DOI: 10.1021/ol050174r). The authors used these chiral salts
in the enantioselective acylation of racemic sec-alcohols with excellent results
(up to 96% ee using 3b).
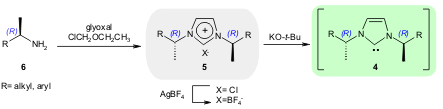
Similar examples of chiral NHCs were also reported by Sako and co-workers.
The NHCs 4 were generated from C2-symmetric
1,3-bis(1-arylethyl)imidazolium salts 5 and potassium tert-butoxide.
The imidazolium salts are readily synthesized from chiral amines 6,
glyoxal, and chloromethyl ethyl ether (Tetrahedron 2006, 62,
302.
DOI: 10.1016/j.tet.2005.09.071). Buy6-Chloroquinoline-2-carboxylic acid Using these catalysts the authors achieved the enantioselective
acylation of racemic sec-alcohols, but with low to modest ee values (up to 68%).
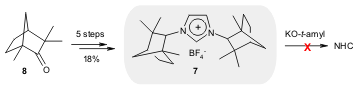
A novel route for the synthesis of imidazolium salt 7 derived from
fenchone (8) was reported by Weaver and co-workers (Org. Lett.
2006, 8, 3049.
DOI: 10.1021/ol0609917). The reported route allows the introduction of
different backbones or substituted backbones into the imidazolium scaffold.
Despite all efforts, the authors were unable to prepare the corresponding NHC
from 7; instead, the product of an unexpected ring expansion was
observed.
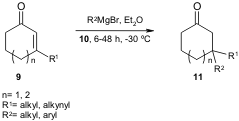
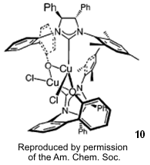
Hoveyda and co-workers published the first examples of Cu-catalyzed
asymmetric conjugate addition (ACA) of alkyl- and arylzinc reagents to simple
unactivated β-substituted
cyclic enones 9 using the chiral NHC-based Cu complex
10 (J. Am. Chem. Soc. 2006, 128, 7182.
DOI: 10.1021/ja062061o). The adducts
11 were obtained in
excellent yields and enantioselection (up to 94% ee).
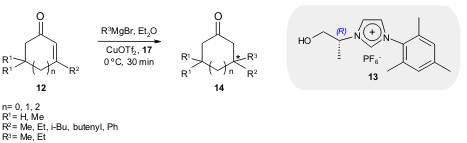
At the same time Alexakis and co-workers also reported the use of chiral NHCs
in the conjugate addition of
Grignard reagents to enones 12 (J. Am. Chem. Soc.
2006, 127, 6284.
DOI: 10.1021/ja0425132). When the chiral imidazolium salt 13 was used, adducts
14 were obtained with excellent yield (93-100%) and moderate to good enantioselection (46-82% ee).
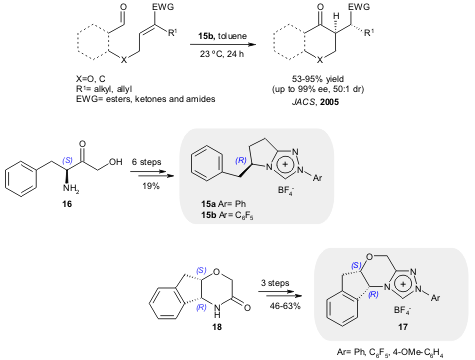
Based on previous work developed in the enantioselective intramolecular
Stetter reaction using NHCs (J. Am. Chem. Soc. 2002, 124,
10298, ;
Synlett 2003, 1934, ; J. Am. Chem. Soc. 2005, 128,
8416,
DOI: 10.1021/ja0629920), Rovis and co-workers
reported an improved synthesis of chiral triazolium salts 15 starting from the
N-Boc protected phenylalanine derivative 16, and the synthesis of
17 starting
from the aminoindanol derivative 18 (J. Org. Chem. 2005, 70, 5725.
DOI: 10.1021/jo050645n).
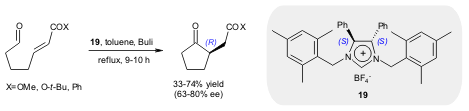
More recently, Tomioka and Matsumoto developed a chiral C2-symmetric NHC from
the chiral dihydroimidazolium tetrafluorobotrate 19 and found that the
asymmetric Stetter reaction proceeds with relatively high enantioselectivity (up
to 80% ee) (Tetrahedron Lett. 2006, 47, 5843.
DOI: 10.1016/j.tetlet.2006.06.080).
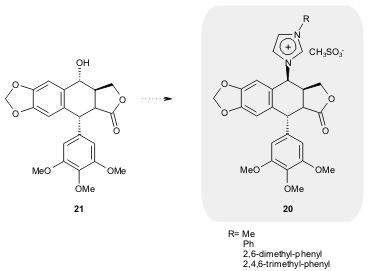
A new class of chiral NHCs 20 was synthesized by Wang and co-workers (62-86%
yield) from the naturally occurring podophyllotoxin 21. The authors used the
corresponding palladium complexes as catalysts in the allylic alkylation
reaction and obtained high conversions (up to 93%) and enantioselectivities (up
to 87% ee) (Tetrahedron: Asymmetry 2006, 17, 1650.
DOI: 10.1016/j.tetasy.2006.06.003).
2. Other Chiral N-Heterocyclic Salts
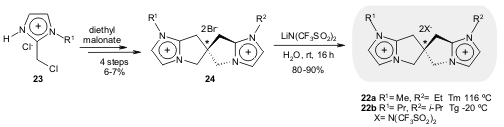
The synthesis of chiral spiro imidazolium salts 22a-b, starting from salts
23
and diethyl malonate, was reported by Sasai and co-workers. Since unsymmetrical
N-substituents contribute to an inefficient crystal packing, the authors
investigated the effect of the N-substituent on the melting point of these
compounds and found that 22b is liquid at room temperature (Tg*= -20 ºC, while
the symmetric analogue has Tm*= 68 ºC). (Org. Lett. 2006, 8, 227.
DOI: 10.1021/ol052466y). A preliminary study of this novel spiro ionic liquid has demonstrated some chiral
discrimination ability.
*Tm= melting temperature
*Tg= glass transition temperature