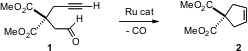Alkene and alkyne metathesis have, in a relatively short time, become important tools for organic synthesis. Carlos Saá of the Universidad de Santiago de Compostela has developed (J. Am. PMID:23927631 Buy4-Amino-2-fluoro-5-methoxybenzoic acid Chem. Soc. 2006, 128, 9576. DOI: 10.1021/ja0610434)a complementary tool for ring construction, the metathetical coupling of an aldehyde or ketone with a terminal alkyne to give the cyclic alkene, illustrated by the conversion of 1 to 2. It seems likely that the oxygen of the CO being expelled comes from the aldehyde, and the carbon comes from the terminal carbon of the alkyne.
1,1-Dibromoalkenes such as 3 are easily prepared from the corresponding aldehyde. Keiji Tanino and Masaaki Miyashita of Hokkaido University have shown (Tetrahedron Lett. 2006, 47, 861. DOI: 10.1016/j.tetlet.2005.12.002)that on exposure of 3 to three equivalents of Me2CuLi, methylation, reduction and intramolecular conjugate addition take place, to give the ester 4 as a 10:1 ratio of diastereomers. 1279032-69-7 web Note that ozonolysis of 4 followed by aldol condensation would give the cyclohexenone 5.
Many methods have been developed for the enantioselective construction of carbocyclic rings, either by cyclization of an enantiomerically-pure precursor, or by asymmetric catalysis. Ken Tanaka of the Tokyo University of Agriculture and Technology has devised (Angew. Chem. Int. Ed. 2006, 45, 2734. DOI: 10.1002/anie.200504470)a third approach. On exposure of the inexpensive racemic 6 to an enantiomerically-pure Rh catalyst, only one enantiomer of 6 reacts, so the product cyclopentenone 7 is delivered in high ee.
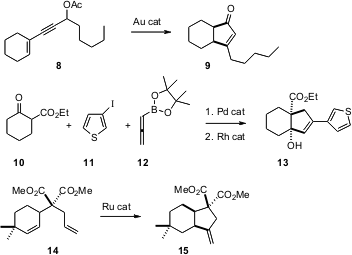
The construction of efficient routes to polycarbocyclic scaffolds for medicinal chemistry exploration is an important objective for organic synthesis. Three useful methods for stitching (“annealing”) a new ring onto a pre-existing ring have recently appeared. Liming Zhang of the University of Nevada, Reno, has found (J. Am. Chem. Soc. 2006, 128, 1442. DOI: 10.1021/ja057327q)an Au catalyst that converts 8, by rearrangement and subsequentNazarov cyclization, to the enone 9. Kenichiro Itami of Nagoya University and Jun-ichi Yoshida of Kyoto University have developed (Org. Lett. 2006,8, 1419. DOI: 10.1021/ol060197l)a Pd-catalyzed three-component coupling of a β-keto ester10, an aryl iodide 11, and the allenylboronate 12. Exposure of the resulting condensation product to Rh catalysis converts it into the cyclized alcohol 13. Ian J.S. Fairlamb of the University of York has extended (Chem. Commun. 2006, 988. DOI: 10.1039/b513798e)Ru-catalyzed cycloisomerization, showing that 14 is converted cleanly to 15. In each of these examples, the cyclization precursors are easily prepared.
Transition-metal mediated cyclizations that directly produce two or more rings offer a powerful entry to polycyclic systems, dramatically reducing the number of steps required for a target-directed synthesis. As exemplified by the recent work (J. Am. Chem. Soc. 2006, 128, 6302. DOI: 10.1021/ja058590u)of Paul A. Wender of Stanford University (16 → 17), a chiral catalyst can effect cyclization and also direct the absolute configuration of the product. In a complementary approach that we have reported (J. Org. Chem. 2006, 71, 2797.DOI: 10.1021/jo052656m), the transition metal, in this case the inexpensive Zr, is used stoichiometrically as a scaffold. Carrying out the cyclization of 18 under equilibrating conditions followed by carbonylation gives the tricyclic ketone 19.