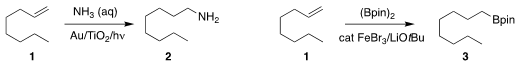
Hisao Yoshida of Kyoto University developed conditions for the direct
hydroamination of the alkene 1 to the
primary amine 2
(J. Am. Chem. Soc. 2020, 142, 12708.
DOI: 10.1021/jacs.0c04598).
Wei Su and Shouxin Liu of the Hebei University of Science and
Technology used an Fe catalyst to effect anti-Markovnikov hydroboration of 1 to
3. Price of 4,6-Dichloro-2-(ethoxymethyl)pyrimidine An alternative Fe catalyst delivered the secondary product (not illustrated)
(ACS Catal. 2020, 10, 11963.
DOI: 10.1021/acscatal.0c02731). Methyl 4-chloro-3-oxobutanoate custom synthesis
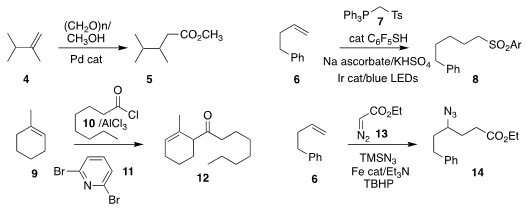
Matthias Beller of the Leibniz- Institut für Katalyse showed that
paraformaldehdye could replace carbon monoxide in the methoxycarbonylation of 4
to the ester 5
(Org. Chem. Front. 2020, 7, 3681.
DOI: 10.1039/D0QO01164A).
Tomoya Miura and Masahiro Murakami, also of Kyoto University, coupled 6
with the phosphonium salt 7 to give the sulfone 8
(Chem. Lett. PMID:34816786 2020, 49, 1382.
DOI: 10.1246/cl.200530).
Shinya Tanaka and Tetsutaro Hattori of Tohoku University observed that the dominant product from the
acylation of 9 with the acid chloride
10 in the presence of the base 11 was the α,γ-unsaturated ketone 12
(Org. Lett. 2019, 21, 8509.
DOI: 10.1021/acs.orglett.9b02688).
Huang Qiu of Sun Yat-sen University and Michael P. Doyle of the University of Texas at San Antonio
assembled the γ-azido ester 14 by coupling 6 with the
diazoacetate 13
(J. Am. Chem. Soc. 2020, 142, 13846.
DOI: 10.1021/jacs.0c05183).
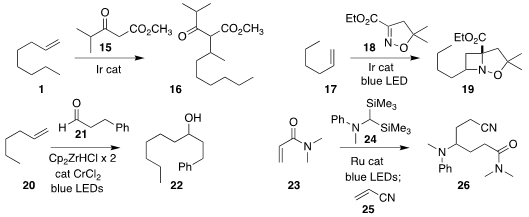
Ryo Takeuchi of Aoyma Gakuin University prepared the β-ketoester 16 by
coupling 1 with the acetoacetate 15
(Org. Lett. 2020, 22, 6187.
DOI: 10.1021/acs.orglett.0c02291).
Corinna S. Schindler of the University of Michigan devised the
photochemical addition of 18 to 17, leading to the
azetidine 19
(Nature Chem. 2020, 12, 898.
DOI: 10.1038/s41557-020-0541-1).
Harunobu Mitsunuma and Motomu Kanai of the University of Tokyo developed the Zr-mediated
direct coupling of the alkene 20 with the aldehyde 21 to give the
secondary alcohol 22
(Org. Lett. 2020, 22, 8584.
DOI: 10.1021/acs.orglett.0c03180).
Lu Gao and Zhenlei Song of Sichuan University used the bis silane 24 as a linchpin,
assembling the α-branched amine 26 by coupling the alkenes 23 and 25
(Chem. Commun. 2020, 56, 7487.
DOI: 10.1039/D0CC02277B).
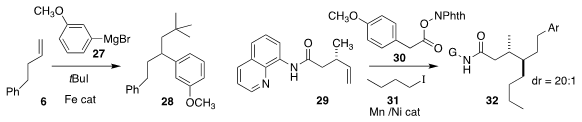
Osvaldo Gutierrez of the University of Maryland employed an iron catalyst to effect
the triply-convergent coupling of 6 with the Grignard reagent 27 and
t-butyl iodide, leading to the α-branched arene 28
(Chem. Sci. 2020, 11, 8301.
DOI: 10.1039/D0SC02127J).
Yu Lan of Chongqin University and Ming Joo Koh of the National University of Singapore
observed high diastereoselectivity in the construction of the amide 32 by the
directed triply-convergent coupling of 29, 30, and 31
(J. Am. Chem. Soc. 2020, 142, 21410.
DOI: 10.1021/jacs.0c09922).
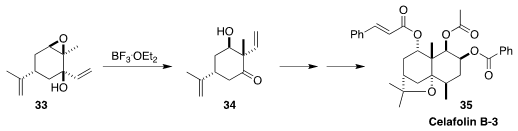
Celafolin B-3 (35), isolated from the South Asian shrub Celastrus flagellaris,
showed significant neuroprotective activity. Yusuke Ogura, also of the
University of Tokyo, established the cyclic alkylated quaternary stereogenic
center of 35 by the rearrangement of 33 to 34
(Org. Lett. 2020, 22, 9234.
DOI: 10.1021/acs.orglett.0c03419).