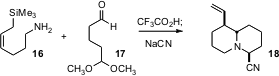Nitrogen heterocycles are the basis of many essential pharmaceuticals, and
of many physiologically-active natural products. There is a continuing interest
in the development of new methods for the construction of nitrogen heterocycles
with control of relative and of absolute configuration. 5-Ethynylpicolinic acid Price
Tom Livinghouse of Montana State University has developed (Org. 1,2,3,4-Tetrahydro-1,5-naphthyridine supplier Lett.
2005,
7, 4391.
DOI: 10.1021/ol051574h)
new Sc-based catalyst systems for intramolecular hydroamination. As
illustrated for the conversion of 1 to 2, the Sc-based catalysts can effect
cyclization with high diastereocontrol. Catalysts based on Zr (Chem. Commun.
2005, 5205.
DOI: 10.1039/b505738h)
are also effective, but so far show low diastereoselectivity.
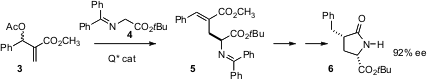
P. Veeraraghavan Ramachandran of Purdue University has shown (J. PMID:23710097 Am. Chem. Soc.
2005, 127, 13450.
DOI: 10.1021/ja052638m)
that a catalyst derived from the Cinchona alkaloids mediates
the phase transfer Michael addition to 3 with high enantio- and geometric
control, giving 5. On deprotection and reduction, 5 is converted
to 6 with high
diastereocontrol.
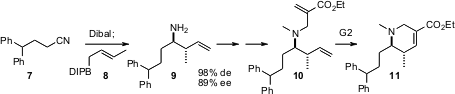
Professor Ramachandran has also (J. Org. Chem. 2005, 70, 7911.
DOI: 10.1021/jo0508200)
taken advantage
of the diastereo-and enantioselective addition of (-)-B-crotyldiisopinocampheylborane
8 to imines developed earlier by the H.C. Brown group. Alkylation of the amine
9 with a Baylis-Hillman adduct followed by cyclization with the second-generation
Grubbs catalyst delivers the dehydropiperidine ester 11.
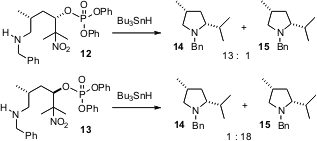
Usually, one would expect that intramolecular trapping of a carbocation would
proceed with little diastereocontrol. David Crich of the University of Illinois
at Chicago has observed (J. Am. Chem. Soc. 2005, 127, 9924.
DOI: 10.1021/ja051657t)
that the radical
cations derived from the reduction of 7 and 8 cyclize predominantly with
inversion of absolute configuration. The radical cation intermediate is
apparently a contact ion pair, with one face blocked by the departing phosphate.
Strategies that allow the controlled construction of multiple ring systems are
also important. Stephen F. Martin of the University of Texas has developed (Org.
Lett. 2005, 7, 2031.
DOI: 10.1021/ol050544b)
a family of allyl silane reagents, represented by
16.
Condensation with the protected dialdehyde 17 leads to the
bicyclic amine
18 as
a single diastereomer.
Many methods have been developed for the diastereoselective and enantioselective
construction of aziridines. Dawei Ma of the Shanghai Institute of Organic
Chemistry has devised conditions (Org. Lett. 2005, 7, 5545.
DOI: 10.1021/ol0513692)
for the
condensation of aziridines with alkynyl esters such as 19. Ring opening and
cyclization proceed with double inversion, to give 21.
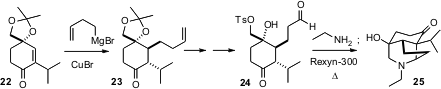
The intramolecular Mannich condensation has been a workhorse of alkaloid
construction. Our group found (J. Org.
Chem. 2005, 70, 8739.
DOI: 10.1021/jo051155y)
that the strong acid-strong base ion exchange resin
Rexyn-300® was effective in pushing a reluctant intramolecular Mannich
condensation to completion.