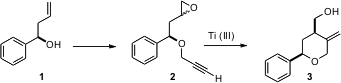
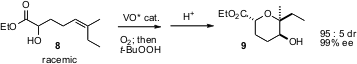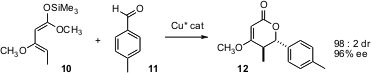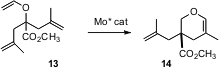Homoallylic secondary alcohols such as 1 are readily prepared in enantiomerically-pure form by enantioselective addition of allyl anions to the aldehyde. Subhas Chandra Roy of the Indian Association for the Cultivation of Science, Jadavpur, has found (Eur. J. Org. Chem. Price of 1374653-45-8 1,2-Benzisoxazol-6-amine Purity 2006, 489.DOI: 10.1002/ejoc.200500424)that the epoxides of the derived propargylic ethers can be cyclized with Ti(III) to the corresponding cyclic ethers. PMID:35954127 The reaction proceeds with high diastereoselectivity, even though the epoxide 2 is a 1:1 mixture of diastereomers.
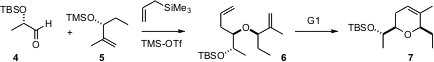
István Markó of the Université Catholique de Louvain has uncovered (Angew. Chem. Int. Ed. 2006, 45, 3357.DOI: 10.1002/anie.200600169)conditions for the highly diastereoselectiveSakurai condensation of an aldehyde such as 4, readily derived in enantiomerically-pure form from ethyl lactate, an enantiomerically-pure secondary silyl ether such as 5, and allyltrimethyl silane. The product ethers 7 are readily cyclized by the first-generation Grubbs catalyst.
5-Exo addition of an alcohol to an epoxide is easy to achieve. 6-Endo addition is much less common. F. Dean Toste of the University of California, Berkeley has reported (Angew. Chem. Int. Ed. 2006, 45, 2096. DOI: 10.1002/anie.200503852)a cascade of enantioselective alcohol oxidation, hydroxy-directed epoxidation by the residual, enantiomerically-enriched alcohol, and finally acid-mediated cyclization, to convert 8 into 9. Yoshiki Morimoto of Osaka City University has described (Angew. Chem. Int. Ed. 2006, 45, 810. DOI: 10.1002/anie.200503143)a complementary approach to 6-endo epoxy alcohol cyclization.
The catalytic establishment of contiguous stereogenic centers with control of both relative and absolute configuration is one of the most sought-after of organic transformations. Xiaoming Feng of Sichuan University in Chengdu has found (J. Org. Chem. 2006, 71, 4141.DOI: 10.1021/jo060046w)that the diene 10 participates efficiently in hetero Diels-Alder cycloaddition with aromatic aldehydes, delivering the adducts 12 in high diastereomeric and enantiomeric excess.
Amir H. Hoveyda of Boston College continues to develop outstanding catalysts for alkene metathesis. Most recently, he has shown (J. Am. Chem. Soc. 2006, 128, 5153.DOI: 10.1021/ja058428r)that an enantiomerically-pure Mo complex will convert prochiral trienes such as 13 to the cyclic ether 14, establishing in the process an otherwise difficult to prepare cyclic quaternary stererogenic center.
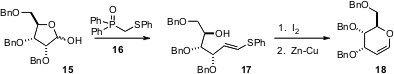
Pyranoid glycals such as 17 are important intermediates for carbohydrate synthesis. Yolanda Díaz and Sergio Castillón of the Universitat Rovira I Virgili, Tarragona, have developed (Org. Lett. 2006, 8, 673. DOI: 10.1021/ol052866l)a general strategy for homologating any pentose, including 15, to the corresponding glycal.
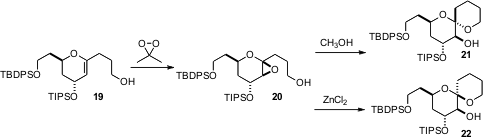
Derek S. Tan of the Memorial Sloan-Kettering Cancer Center, New York, has been extensively investigating (J. Am. Chem. Soc. 2006, 128, 1792. DOI: 10.1021/ja057908f)the stereocontrolled construction of spiro ethers such as 20 and 21. From the same glycal epoxide 19, either 20 or 21 can be formed, depending on the acid used. An obvious question is whether an alkylated version of 16 could be used to prepare 1-alkyl glycals such as 19.