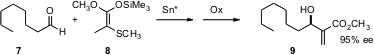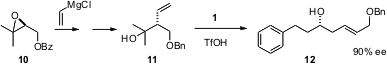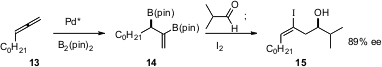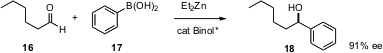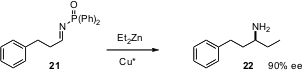Convergent coupling of a nucleophile with an aldehyde ideally will proceed with high enantioselectivity, while at the same time allowing the attachment of a usefully functionalized fragment. Several recently-reported aldehyde homologations are particularly noteworthy.
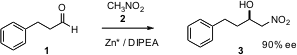
One of the simplest of functionalized nucleophiles is nitromethane (2). Claudio Palomo of the Universidad del País Vasco has found (Angew. Chem. Int. Ed. 2005, 44, 3881. 17193-29-2 supplier DOI: 10.1002/anie.200463075)that a Lewis acid and an amine base together mediate the enantioselective addition of nitromethane to an aldehyde 1 (Henry reaction) to give the alcohol 3.
The enantioselective addition of an alkyne 4 to an aldehyde 5 has become a workhorse of organic synthesis. Masakatsu Shibasaka of the University of Tokyo has developed (J.Am. Chem. Soc. 2005, 127, 13760. 4-Chloro-5-methoxypyridin-2-amine Chemscene DOI: 10.1021/ja053946n)an indium-based protocol for this addition.
Isamu Shiina of the Tokyo University of Science has devised (J. Org. Chem. PMID:24257686 2005, 70, 8103. DOI: 10.1021/jo051276y)an enantioselective procedure for the three-carbon homologation of an aldehyde 7 to give, after oxidation of the initial adduct, the unsaturated ester 9 (Baylis-Hillman reaction). Note that this transformation should work equally well with esters bearing longer sidechains than 8.
Acid-catalyzed allyl transfer is an efficient method for adding an allylic “anion” to an aldehyde with control of regioselectivity, geometry and absolute configuration. Junzo Nokami of the Okayama University of Science has introduced (Org. Lett. 2005, 7, 2957. DOI: 10.1021/ol050890t)a new route to substituted allyl donors, based on alkenyl addition to 10, prepared bySharpless asymmetric epoxidation. The ee of 12 is the same as the ee of 10. It might be possible to devise a derivative of 10 or of 11 that could be recrystallized to higher ee.
One of the most powerful methods for catalytic enantioselective aldehyde homologation ever developed is the catalytic asymmetric allene diboration approach reported (Org. Lett. 2005, 7, 5505. DOI: 10.1021/ol052312i)by James P. Morken, now at Boston College. Sequential reaction of 14 with an aldehyde and then with another electrophile leads to highly functionalized, stereodefined products such as 15.
The catalytic enantioselective addition of an aryl nucleophiles to aldehydes has been difficult to achieve. Katsuji Ito of the Fukuoka University of Education and Tsutomu Katsuki of Kyushu University have reported (Tetrahedron Lett. 2005, 46, 6083. DOI: 10.1016/j.tetlet.2005.06.161)a general solution to this problem, using an areneboronic acid activated by Et2Zn in presence of a catalytic amount of a binol derivative.
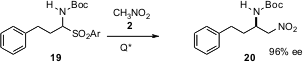
Alkyl imines do not react with nucleophiles such as 14, so more electrophilic imine equivalents have been developed. Independently, Raquel P. Herrera and Luca Bernardi of the Università di Bologna (Angew. Chem. Int. Ed. 2005, 44, 7975. DOI: 10.1002/anie.200502646)and Professor Palomo (J. Am. Chem. Soc. 2005, 127, 17622. DOI: 10.1021/ja056594t)have described the use of α-sulfonyl amine derivatives such as 19. addition of nitromethane in the presence of a chiral quaternary ammonium salt delivered the aza-Henry product 20.
The multicomponent condensation created by André Charette of the Université de Montreal (J. Org. Chem. 2005, 70, 10864.DOI: 10.1021/jo0516483)is particularly convenient. Exposure of the phosphonyl imine 21, prepared in situ, to a dialkyl zinc in the presence of a chiral Cu catalyst gives, after workup, the unprotected primary amine 22 in high ee.