Powerful methods for the stereocontrolled construction of cyclic ethers have recently been developed, enabling the synthesis of a variety of complex natural products.
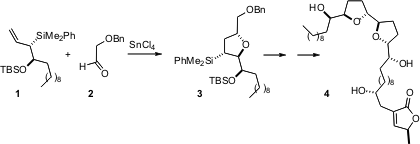
Many of the Annonaceous acetogenins, represented by asimicin (4), exhibit powerful and selective cytotoxicity against human malignant cell lines. Fmoc-leucine Chemscene William R. Roush, now at Scripps Florida, has developed (J. Boc-NH-PEG4-CH2CH2NH2 custom synthesis Am. Chem. PMID:23664186 Soc. 2005, 127, 10818.DOI: 10.1021/ja051986l)a method for the stereocontrolled construction of trisubstituted tetrahydrofurans such as3, based on the condensation of enantiomerically-pure allyl silanes such as 1 with aldehydes. It is particularly elegant that by the choice of Lewis acid, the combination of 1 and 2 can be directed cleanly to either the trans product 3 or the cis diastereomer. This condensation was applied twice in the synthesis of asimicin (4).
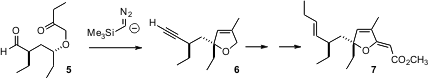
The ester 7 is representative of several closely-related natural products isolated from the Caribbean sponge Plakortis halichondroides. The central challenge in the synthesis of 7 is the stereocontrolled assembly of the quaternary center. Susumu Ohira of the Okayama University of Science has put forward (Tetrahedron Lett. 2005, 46, 7483.DOI: 10.1016/j.tetlet.2005.09.011)an elegant solution to this problem, based on the cyclization of the ketone 5. Using the lithium salt of TMS diazomethane, the ketone and the aldehyde are each converted into the corresponding alkylidene carbene. The alkylidene carbene from the aldehyde spontaneously rearranges into the terminal alkyne. The carbene derived from the ketone effects concerted intramolecular C-H insertion into the proximal ternary center of 5, establishing the quaternary center of 6 and thus of 7.
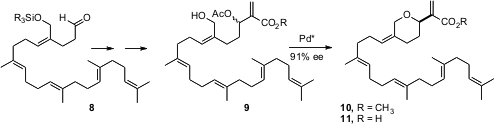
Barry M. Trost of Stanford University has extensively developed Pd-catalyzed dynamic kinetic asymmetric transformation (DYKAT) as a tool for organic synthesis. The power of this method is illustrated by the enantioselective synthesis (J. Am. Chem. Soc. 2005, 127, 7014.DOI: 10.1021/ja050340q)of the gastrulation inhibitor (+)-hippospongic acid A (11). The Baylis-Hillman adduct 9 is racemic, but the enantiomerically-pure Pd complex converts it into 10 in high ee.
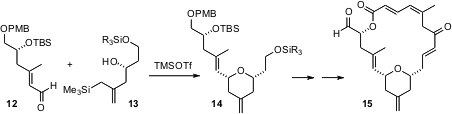
As illustrated in the synthesis of (+)-dactylolide reported (Org. Lett. 2005, 7, 3053.DOI: 10.1021/ol051040g)by Gary E. Keck of the University of Utah, 2,6-dialkyl tetrahydropyran derivatives can be prepared with high diastereocontrol under equilibrating conditions. The secondary alcohol of 13 sets the relative and absolute configuration of14. Both 12 and 13 were prepared using the catalytic enantioselective allyl stannane addition developed by Professor Keck.
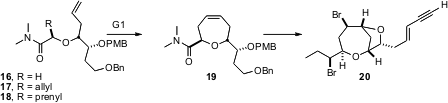
In the course (J. Org. Chem. 2005, 70, 8723.DOI: 10.1021/jo050974f)of a synthesis of (-)-isoprelaurefucin (20), a metabolite of Laurencia nipponica, Deukjoon Kim of Seoul National University took advantage of remarkable diastereoselectivity in the alkylation of the chelated lithium enolate derived from 16. Allylation proceeded in a 10:1 diastereomeric ratio, to give 17, and prenylation proceeded in a 15:1 ratio, to give 18. Exposure of 18 to the first generation Grubbs catalyst delivered19.