Matthew J. Formula of 6-Bromo-2-oxaspiro[3.3]heptane Pelc and Armen Zakarian from Florida State University have reported
on the synthesis of A,G-spiroimine (1) of pinnatoxins (Tetrahedron Lett.
2006, 47,
7519. Sodium difluoromethanesulfinate Order
DOI: 10.1016/j.tetlet.2006.08.083). Key step in this procedure is a cascade sigmatropic route that
integrates the Claisen and Mislow-Evans rearrangements. By applying
microwave-heating at 170 °C, the reaction time could be reduced to 20 min
compared to 15 h under conventional heating at 150 °C.

Synthesis of Functionalized 1-Amido-2-cyclohexenes
An improved protocol for the three-component synthesis of aldehydes, amides and
dienophiles (AAD-reaction) was developed by the group of Matthias Beller from
the University of Rostock (Tetrahedron 2006, 62, 10962.
DOI: 10.1016/j.tet.2006.08.062). PMID:34337881 Optimization
studies revealed that a solventless microwave protocol (150 °C, 20 min) gave a
superior yield (90 vs 61%) for the reaction of acetamide (1), crotonaldehyde (2)
and N-methyl-maleimide (3) in a 50 times shorter reaction time compared to
thermal heating at 110 °C. The addition of acetic acid anhydride (Ac2O) as water
removing reagent proved to be beneficial for the reaction. All 10 cyclohexene
derivatives are obtained as only one diastereomer, namely as the all-syn product,
due to the selective endo addition of the dienophile.

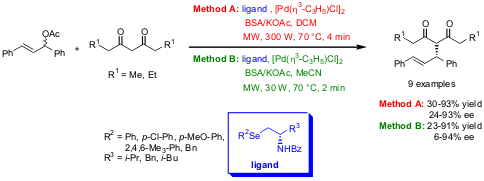
Ru-Catalyst on Raschig Rings for Olefin Metathesis
The groups of Andreas Kirschning (Universität Hannover) and Karol Grela (Polish
Academy of Science) have developed the Ru-catalyst 1 for various metathesis
reactions (J. Am. Chem. Soc. 2006, 128, 13261.
DOI: 10.1021/ja063561k). The Ru-complex is
noncovalently (ion exchange) immobilized on glass-polymer composite Raschig
rings which has the advantage of easy removal and reactivation after the
reaction. By applying microwave heating, the reaction could be enhanced from 2 h
to 4 min but with slightly reduced isolated yields. Additionally to the batch
protocol, the olefin metathesis was also performed under PASSflow continuous
flow conditions, however, a diminished recyclability of
1 (6 cycles vs 2) was observed.
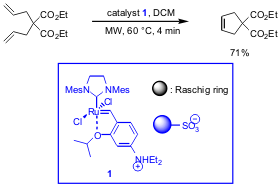
Pd-Catalyzed Asymmetric Allylic Alkylations
The group of Antonio L. Braga from Universidade Federal de Santa Maria, Brazil,
has disclosed Pd-catalyzed allylic alkylations of rac-1,3-diphenyl-2-propenyl
acetate with malonates (Eur. J. Org. Chem. 2006, ASAP.
DOI: 10.1002/ejoc.200600707). By applying chiral
β-seleno amides as ligands in combination with microwave heating, reaction times
of 2-4 min were possible compared to 24 h at rt with only a minimal loss of
enantioselectivity (ee´s up to 94% were obtained). During optimization studies,
two methods were found to give high yields (method A and
B). For ligands with R2
= Ph and R3 = Bn, i-Bu it turned out that the method which is applied has a
significant influence on the ee´s, better values could be reached with method
A.