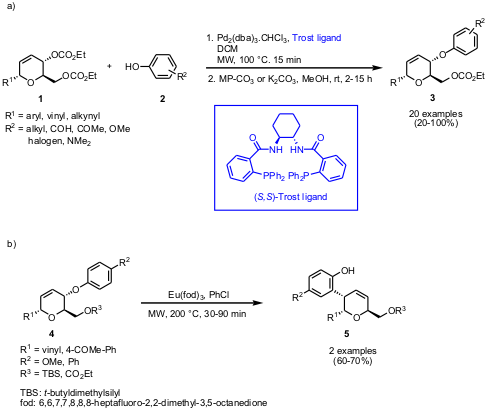
The group of John A. Porco, Jr. and Scott E. Schaus from Boston University has
reported on the synthesis of aryl ether C-glycoside derivatives 3 via
Pd(0)-mediated allylic substitution of biscarbonates 1 with phenols 2
(Org. (S)-(-)-3-Butyn-2-ol Chemical name EPhos Pd G4 In stock Lett. 2006, 8, 5065.
DOI: 10.1021/ol0618252). PMID:23829314 This
reaction was both regio- and stereoselective with an overall retention of
configuration when the enantiomeric (S,S)-Trost ligand was employed. The
protected aryl ether C-glycosides 4 can be further diversified by
Eu(III)-catalyzed Claisen rearrangement where glycosides 4 undergo a
[3,3]-sigmatropic rearrangement and furnish the novel phenol derivatives 5.
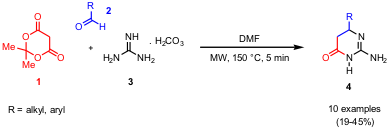
Synthesis of 2-Amino-dihydropyrimidin-4-ones
The three-component reaction of Meldrum´s acid (1),
aldehydes 2 and guanidine carbonate (3)
for the simple synthesis of 2-amino-dihydropyrimidines 4 was developed by
Nikolay Yu. Gorobets and co-workers from the National Academy of Scienes of
Ukraine (Mol. Diversity 2006, 10, 483.
DOI: 10.1007/s11030-006-9045-1). The reactions
were performed both under conventional (120-130 °C, 20-25 min) and microwave
heating (150 °C, 5 min) showing slightly higher yields for most of the derivates
applying conventional conditions. Importantly, to avoid high pressure build up
in the closed microwave vials due to CO2 formation, the reaction
mixture was stirred for 10-15 min at rt before subjecting to microwave
irradiation.
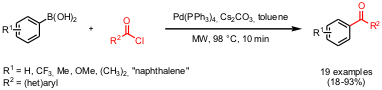
Cross-Coupling of Arylboronic Acids with Acid Chlorides
A set of 19 symmetrical and unsymmetrical aryl ketones was prepared via
Suzuki coupling of arylboronic acids with acid chlorides in moderate to high
yields under very mild reaction conditions (98 °C, 10 min) by the group of Viera
Poláčková from Comenius University in Bratislava (Tetrahedron 2006,
62, 11675.
DOI: 10.1016/j.tet.2006.09.055). A catalyst screen
for the reaction of phenylboronic acid and 4-nitrobenzoyl chloride revealed that Pd(PPh3)4
and Cs2CO3 as catalyst/base system provided the aryl
ketone (R1 = Ph, R2 = 4-NO2-Ph) in highest
yield under the given microwave-conditions.
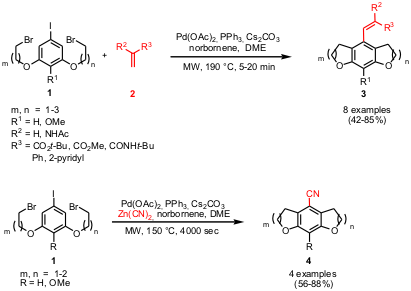
Synthesis of Tricyclic Symmetrical and Unsymmetrical Heterocycles
Mark Lautens and co-workers from the University of Toronto have investigated
the synthesis of tricyclic heterocycles (3 and 4) starting from
aryl iodide 1 which contains two tethered alkyl bromides and a Heck
acceptor 2 or Zn(CN)2,
respectively. Products 3 are obtained via a norbornene-mediated
Pd-catalyzed intramolecular bis-alkylation/intermolecular alkenylation sequence
of 1 and 2 (Synlett 2006, 2969.
DOI: 10.1055/s-2006-941583)
whereas tricyclic benzonitriles 4 are received via tandem intramolecular
bis-alkylation/intermolecular cyanation reaction applying the same catalytic
system (J. Am. Chem. Soc. 2006, 128, 14436.
DOI: 10.1021/ja064742p).