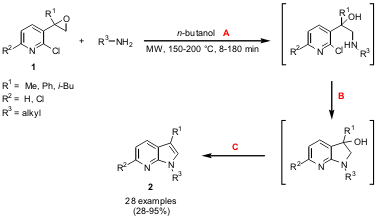
Hartmut Schirok from Bayer HealthCare, Germany, has reported on a short and flexible synthesis of 1,3-substituted 7-azaindoles 2 starting from oxiranes 1 (J. PMID:23724934 Org. Chem. 2006, 71, 5538.DOI: 10.1021/jo060512h). The reaction of epoxides 1 with a variety of substituted primary amines proceeds via an epoxide-opening (A)/cylization (via SNAr, B) /dehydration (C) sequence, which is accelerated by microwave irradiation. 89284-85-5 web The higher temperature (200 °C) was required for the mono-chloro pyridine derivatives (R2 = H) since they proved to be less reactive. 1,12-Dibromododecane Formula As opposed to the conventional heating protocol, no addition of an acid was required for the dehydration step.
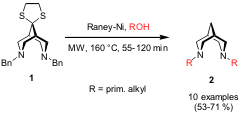
Raney Nickel Reductions
In a recent publication, Adolf Gogoll and co-workers from Uppsala University have developed the Raney nickel catalyzed reduction of thiolane 1 to N,N’-dialkyl-3,7-diazabicyclo[3.3.1]nonanes (bispidines) 2 (Synthesis2006, 2064.DOI: 10.1055/s-2006-942394). Primary alcohols, which also serve as reagents and to which the reaction is limited, proved to be the best solvents. After initial thioacetal reduction and simultaneous debenzylation, the generated secondary amine is alkylated by the alcohol, which was converted to the corresponding carbonyl compound. Best reaction conditions to reach full conversion turned out to be 20 mass equiv of Raney nickel, a temperature of 160 °C and 55-120 min as reaction time (compared to two days under conventional reflux heating).
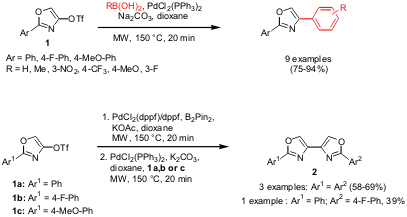
Suzuki Coupling of Oxazoles
The group of Michael F. Greaney from the University of Edinburgh has described a protocol for the arylation of oxazoles in the 4- or 2-position, respectively, via Suzuki reactions (Org. Lett. 2006, 8, 2495.DOI: 10.1021/ol060591j). 2-Aryl-4-trifloyl oxazoles 1 were coupled with a variety of boronic acids, whereas the coupling at the 2-position was performed with 2-chloro-4-phenyl oxazole as coupling partner. Both couplings were conducted at the same reaction conditions (5 mol% PdCl2(PPh3)2, Na2CO3, dioxane, 150°C, 20 min). The reaction was further extended to the one-pot synthesis of dioxazoles. In the first step, boronic esters were generated in situ via the reaction of 1a-c with bis(pinacolato)diboron (B2Pin2), in the next sequence, 1 equiv of 1a,b or c was added to the reaction mixture together with the reagents needed for the Suzuki coupling (catalyst, base). In this way, homo- and heterodimeric dioxazoles 2 were obtained.
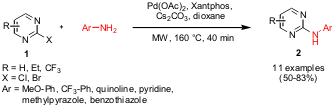
Pd-Catalyzed Synthesis of 2-Arylaminopyrimidines
Henry Q. Zhang and co-workers from Abbott Laboratories, Abbott Park, have shown that 2-chloro pyrimidines 1 can be converted to the corresponding 2-aryl or 2-heteroaryl pyrimidines 2, respectively, via Pd-mediated aminations (Tetrahedron Lett. 2006, 47, ASAP,DOI: 10.1016/j.tetlet.2006.05.036). The reactions were conducted under both Pd-free conventional and microwave heating as well, furnishing the 2-aryl pyrimidines in similar yields as under Pd-catalyzed microwave conditions. However, when 2-heteroaryl amines are reacted with 1, only the Pd-catalyzed microwave protocol afforded the desired products. 2-Bromo pyrimidines were also found to react under these conditions.