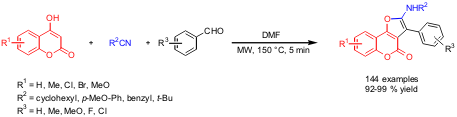
A 144-member library of furocoumarins via three-component reaction of 4-hydroxycoumarins, isocyanides and arylaldehydes was prepared by Jie Wu from VivoQuest Inc., NY (Chem. Lett. 2006, 35, 118. (4-Chlorophenyl)(2-nitrophenyl)sulfane web DOI: 10.1246/cl.2006.118). By applying a microwave protocol (DMF, 150 °C, 5 min), reduction in reaction times (24 h → 5 min), higher yields and less by-products were observed compared to the conventional synthesis. PMID:35850484
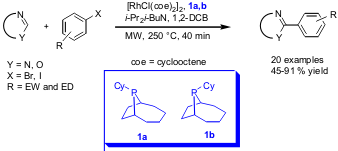
Rh-Catalyzed Arylation of Heterocycles
Jonathan Ellman and co-workers from the University of California, Berkeley, have reported on the direct coupling of azoles with aryl bromides and iodides, respectively by Rh-catalyzed C-H bond activation (Angew. Chem. Int. Ed. 1350518-27-2 structure 2006, 45, in advance of print. DOI: 10.1002/anie.200504289). Superior yields could be obtained by using a mixture of the bulky trialkylphosphines 1a and 1b as ligand compared to PCy3 due to less hydrodehalogenation. A variety of functional groups in para and meta position were tolerated and the method was also compatible with different heterocycles.
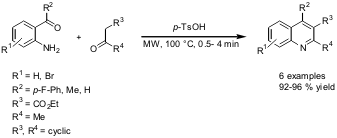
Friedländer Annulation
The synthesis of poly-substituted quinolines applying the Friedländer condensation was disclosed by the group of Guan-Wu Wang from the University of Science and Technology of China, Hefei (Org. Biomol. Chem. 2006, 4, 104. DOI: 10.1039/b513721g). By conducting the reaction under microwave-solvent-free conditions withp-toluene sulfonic acid (p-TsOH) as catalyst, the quinoline products could be obtained in excellent yields through a simple work-up procedure. Identical results were achieved performing the reactions under conventional heating at the same temperature, but with prolonged reaction times.
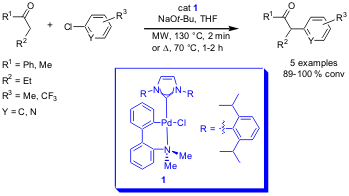
α-Ketone Arylation
Comparison studies of microwave vs. conventional heating in the arylation of α-ketones with aryl chlorides have been performed by Steven Nolan and his group from the University of New Orleans (J. Org. Chem. 2006, 71, 685. DOI: 10.1021/jo0521201). By employing the versatile N-heterocyclic carbene palladacycle 1 as catalyst, full conversion was reached under both microwave and conventional conditions. Due to the higher temperatures (130 vs. 70 °C) which can be reached by microwave heating, the reactions proceeded up to 60 times faster.