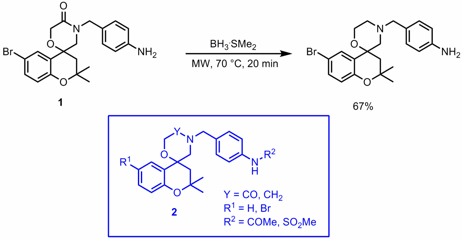
Mohammad Movassaghi and Matthew D. Hill from MIT have developed a protocol for the synthesis of quinazolines 1 via electrophilic activiation of secondary amides utilizing 2-chloropyridine (2-ClPyr) in combination with Tf2O and subsequent reaction with weakly nucleophilic nitriles (J. Am. Chem. Soc. 2006, 128, 14254. DOI: 10.1021/ja066405m). PMID:23962101 Whereas electron richN-vinyl and -aryl amides afforded the corresponding pyrimidine derivatives at room temperature, microwave heating to 140 °C was necessary for less reactive substrates. Dibenzyl carbonate manufacturer 1240587-95-4 uses Additionally, one example was presented where a primary amide was employed instead of the nitrile under the same microwave conditions giving the quinazoline (R1 = Ph, R2 = H, R3 = OMe, R4 = cC6H11) in 74% yield.
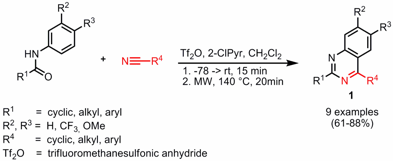
Synthesis of Azido-Modified Silica Gels
Wolfgang Lindner and co-workers from the University of Vienna have reported on the nucleophilic halide/azide exchange of pre-activated silica gels 1 (Tetrahedron Lett. 2006, 47, 8721.DOI: 10.1016/j.tetlet.2006.10.018). By applying microwave heating the reaction time could be reduced from 48 h at 80 °C to only 5 min at 125 °C. For the azidopropyl-modified silica support 2a an 87% and for the 11-azidoundecyl silica2b even quantitative conversion was obtained. The azido-modified silica supports were then subjected to click chemistry with alkyne-functionalized Cinchona alkaloids 3 and 4, respectively, in order to produce an effective chiral stationary phase for HPLC enantiomer separation.
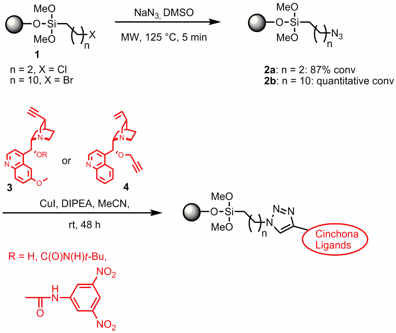
Reduction of Spiromorpholone
The groups of Vincenzo Calderone and Simona Rapposelli from the Università di Pisa have disclosed the microwave-assisted reduction of spiromorpholone 1 with the borane-dimethyl sulfide complex BH3•SMe2 in the course of the synthesis of conformationally restricted benzopyrans 2 by the introduction of a spirocyclic substituent at the C-4 position (J. Med. Chem. 2006, 49, ASAP.DOI: 10.1021/jm061228l). It was found that some compounds possessing this spirocyclic-type of substitution exhibit potent anti-ischemic properties without affecting the blood pressure parameters.
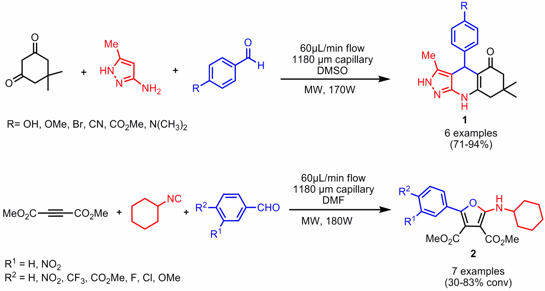
Continuous Flow Synthesis of Heterocycles by Multicomponent Reactions
Michael G. Organ and W. Stacey Bremner have employed microwave-assisted continuous flow organic synthesis (MACOS) for the preparation of heterocyclic compounds via multicomponent reactions utilizing a self-designed capillary system as reactor (J. Comb. Chem. 2006, 8, ASAP.DOI: 10.1021/cc060130p). The three components are each introduced into the microwave cavity through three separate leads, in equal concentrations and at the same flow rate. In this way a set of tetrahydropyrazolo[3,4-b]quinolin-5(6H)-ones 1 and aminofurans2, respectively, could be accomplished in a few seconds and in very good conversions. Importantly, this flow system is an open system which means that high boiling and good microwave-absorbing solvents like DMSO or DMF have to be used to reach higher temperatures and hence higher conversions.