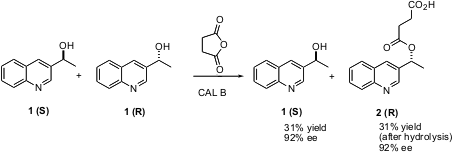
Enzymes have many applications in organic synthesis. One of the most common is the resolution of a secondary alcohol. A shortcoming of this approach is that the separation of the residual alcohol from the product ester has required column chromatography, adding to the expense. Louisa Aribi-Zouioueche of the University of Annaba, Algeria, and Jean-Calude Fiaud of the University Paris-Sud, Orsay, have been exploring (Tetrahedron Lett. 2004, 45, 627.DOI: 10.1016/j.tetlet.2003.10.208)the use of succinic anhydride as an alternative to the more typical vinyl acetate or isopropenyl acetate acylating agents. 6-Chloro-1,5-naphthyridin-2(1H)-one In stock Using this procedure, the residual alcohol and the product ester can be separated by simple acid-base extraction. PMID:24268253 The key question they had to address was whether or not the enantioselectivity of the acylation was maintained. 1378254-82-0 web The results were mixed, but in at least one case, the quinoline alcohol1, the enantioselectivity was improved using this protocol.
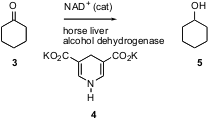
Enzymes can also be used to reduce organic substrates, as illustrated by the conversion of cyclohexanone 3 to cyclohexanol 5. A shortcoming of this approach is that a stoichiometric amount of the reducing cofactor, in this case NADH, is required. This need can be met by simultaneously oxidizing a sacrificial substrate, so as to regenerate the NADH. Ikuo Ueda of Osaka University has developed (J. Org. Chem. 2002, 67, 3499.DOI: 10.1021/jo025525j)an interesting alternative. The inexpensive Hantzsch carboxylate 4 will regenerate NADH from NAD+. Using this procedure, they were able to observe up to ten turnovers of the cofactor.
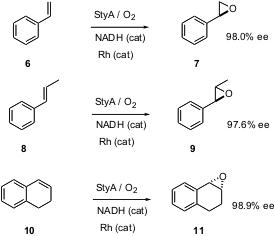
It is interesting that NADH is also required as a stoichiometric co-factor in enzymatic oxygenation processes. In a detailed study of styrene monooxygenase (StyA), Andreas Schmid of the ETH / Zurich showed (J. Am. Chem. Soc. 2003, 125, 8209.DOI: 10.1021/ja034119u)that Cp*Rh(bpy)(H2O)2+ in combination with sodium formate served effectively to regenerate the NADH. Using this combination, epoxidation of aryl alkenes such as6, 8 and 10 proceeded in high enantiomeric excess.