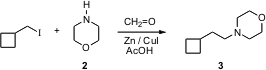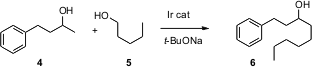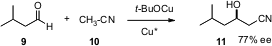Carbon-carbon bond formation is the fundamental transformation of organic synthesis. Several new procedures have been put forward that offer advantages of selectivity and economy. 2409005-96-3 manufacturer
Lothar W. PMID:23724934 Beiber of the Universidade Federal de Pernambuco, Recife, Brazil, has reported (Tetrahedron Lett. 2005, 46, 7601.DOI: 10.1016/j.tetlet.2005.08.139)the one-carbon aminoalkylation of alkyl halides such as 1 using Zn and acetic acid. Remarkably, aqueous formaldehyde works efficiently in the reaction.
The telescoping of several synthetic steps offers substantial advantages on scale. Ken-ichi Fujita and Ryohei Yamaguchi of Kyoto University have developed (Org. Lett. 2005, 7, 4017.DOI: 10.1021/ol051517o)an Ir catalyst that mediates the condensation of a secondary alcohol such as 4 with a primary alcohol such as 5. The overall transformation must include six steps: oxidation of each alcohol, aldol condensation, dehydration, reduction of the alkene, and reduction of the ketone. 1258874-29-1 site
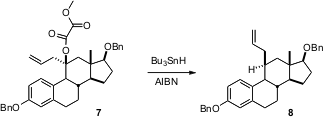
One might think that ternary alkyl centers such as that of 8 would be formed by nucleophilic substitution. Jean-Claude Blazejewski of the Université de Versailles has described (J. Org. Chem. 2005, 70, 8907.DOI: 10.1021/jo051424k)an alternative approach, free radical reduction of the corresponding tertiary alcohol. Equatorial H atom addition sets the relative configuration of the pendant alkyl group. With radical-stabilizing substituents, an increasing proportion of the equatorial alkyl group is observed.
The anion of acetonitrile 10 adds efficiently to aldehydes and ketones. Simultaneously, Oleg. V. Ozerov of Brandeis University (Chem Commun. 2005, 4450. DOI: 10.1039/b505778g)and Masakatsu Shibasaki of the University of Tokyo (Chem Commun. 2005, 3600. DOI: 10.1039/b504519c)reported that this addition can be carried out using sub-stoichiometric DBU and a transition metal catalyst. Later, Professor Shibasaki and his colleague Motomu Kanai found (Org. Lett. 2005, 7, 3757.DOI: 10.1021/ol051423e)that the use of a chiral Cu catalyst led to the adduct with useful ee.
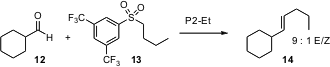
The Julia olefination using heteroaryl sulfones has been developed extensively over the past several years. Carmen Nájera of the Universidad de Alicante has now established (J. Org. Chem. 2005, 70, 6404.DOI: 10.1021/jo050852n)that electron-withdrawing arene sulfones such as 13 in conjunction with phosphazene bases also work well.
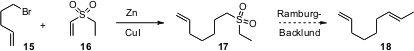
Matthew M. Zhao of Merck Process, Rahway, has found (J. Org. Chem. 2005, 70, 6944.DOI: 10.1021/jo050500g)that Zn/Cu mediates the addition of halides to unsaturated sulfones such as 16. Unsaturated sulfonates work well also. Note that Ramberg-Bäcklund contraction of the sulfone 17 would give the alkene 18.
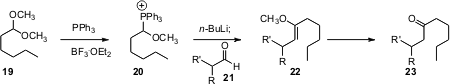
Steven D. Burke of the University of Wisconsin reports (J. Org. Chem. 2005, 70, 9382.DOI: 10.1021/jo051479m)a simple procedure for the homologation of an aldehyde such as 21 to the ketone 23. The phosphonium salt 20 was readily prepared from the acetal. The key to this approach was to convert 20 to the phosphorane at -40°C.