Nitrogen heterocycles, because of their relative ease of preparation, have dominated pharmaceutical discovery. Improved methods for the diastereoselective and enantioselective construction of oxygen heterocycles have now made these much more readily available. 1-(p-Tolylsulfinyl)bicyclo[1.1.0]butane Chemical name
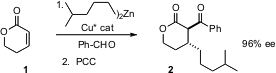
Unsaturated lactones such as 1 and butenolides as five-membered ring analogue are inexpensive. Attempts at enantioselective conjugate addition to these very reactive receptors have been plagued by low material recovery. Amir H. Hoveyda of Boston College speculated (Angew. Chem. Int. Ed. 2005, 44, 5306. PMID:24605203 DOI: 10.1002/anie.200501251)that the problem lay with the reactivity of the intermediate lactone enolate. He included a trapping aldehyde in the reaction mixture, and found that product ee was outstanding, and the mass balance was substantially improved.
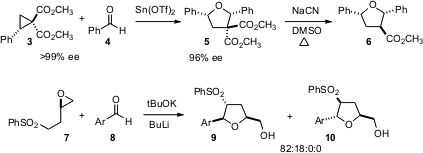
Many methods have been developed for the stereocontrolled synthesis of five- and six-membered ethers using SN2 displacement. Even more interesting are recent observations of high stereoselectivity where that might not have been expected. Jeffrey S. Johnson of the University of North Carolina has found (J. Am. Chem. Soc. Buy4-Chloropyrimidine-2-carbonitrile 2005, 127, 16014.DOI: 10.1021/ja055777c)that the opening of the activated cyclopropane 3 proceeds via an incipient carbocation, that is trapped by the nonbonding electrons of the aldehyde 4 with nearly perfect inversion, leading to 5. Even more striking is the coupling of 7 and 8 to give predominantly 9, reported (Org. Lett. 2005, 7, 2945.DOI: 10.1021/ol0509080)by Mieczyslaw Makosza of the Polish Academy of Sciences, Warsaw. Apparently, the initial aldol condensation is reversible, as demonstrated by crossover experiments with an isolated intermediate put back into the reaction. Of the four diastereomeric aldol products, only two do the intramolecular epoxide opening, one significantly more rapidly than the other. The absolute configuration of 9 and of 10 is set by the absolute configuration of the starting epoxide 8.
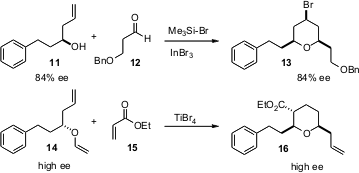
The Prins cyclization has become a workhorse of six-memberedcyclic ether construction. Teck-Peng Loh of Nanyang Technological University, Singapore, observed (Org. Lett. 2005, 7, 4491.DOI: 10.1021/ol051951q)that allyl adducts such as 11 tended to racemize before cyclizing. Use of InBr3 as the catalyst suppressed racemization, delivering 13 in high ee. In related work, Scott D. Rychnovsky of the University of California, Irvine has reported (J. Am. Chem. Soc. 2005, 127, 16044.DOI: 10.1021/ja056483u)an elegant Mukaiyama-Michael cascade protocol. The formation of 16 proceeds with inversion of absolute configuration, but maintains the enantiomeric purity of the starting ether 14.
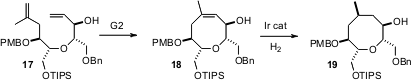
As illustrated by the recent work (Org. Lett. 2005, 7, 4033. DOI: 10.1021/ol051543m)of Michael T. Crimmins of the University of North Carolina, medium rings can often be formed efficiently byGrubbs metathesis. With the proper choice of catalyst,18 can be hydrogenated to either diastereomer of 19.
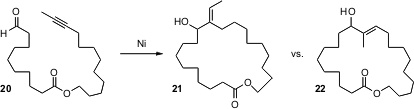
Attempted formation of larger rings often leads to unwanted polymer formation. John Montgomery, now at the University of Michigan, has shown (J. Am. Chem. Soc. 2005, 127, 13156.DOI: 10.1021/ja054590i)that with the proper Ni catalyst, even large rings can be formed efficiently. Remarkably, with the proper choice of reaction conditions, the cyclization can be directed to either 21 or 22.