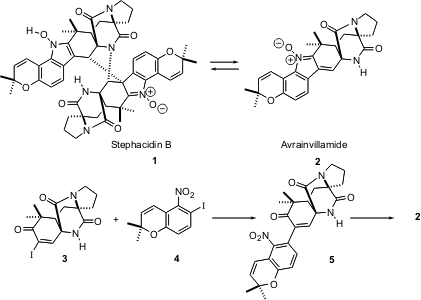
The dimeric alkaloid stephacidin B (1) was recently isolated from a
fungus culture. The “monomer” avrainvillamide (2) had previously been
described. Andrew G. Myers of Harvard University has reported (J. Am. Chem.
Soc. 1097871-14-1 Formula 1-(3-Aminopropyl)azepan-2-one supplier 2005, 127, 5342.
DOI: 10.1021/ja0510616)
the enantioselective total
synthesis of 2, and the dimerization of 2 to 1. PMID:25429455 The key
intermediate in the synthesis was the tetracyclic amide 3.
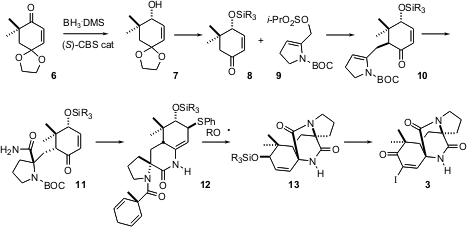
The absolute configuration of the target natural products was set by
enantioselective reduction of the enone 6. Usually, catalytic
Itsuno-Corey
reduction of cyclohexenones without an α-substituent is not selective. In this
case, advantage was taken of that lack of induction from the alkene side, with
the steric bulk on the other side of the ketone directing the reduction.
Alkylation of 8 with 9 proceeded to give the expected axial product
10.
Cyanation proceeded with remarkable diastereocontrol, to give, after
epimerization and hydrolysis, the amide 11. Conjugate addition of thiophenol
followed by spontaneous cyclization and dehydration led to the amide 12. With
the phenylthio enamide in place, the stage was set for the elegant final
cyclization: hydrogen atom abstraction from the dihydroaromatic followed by
fragmentation delivered the formamide radical, that cyclized efficiently to give
the tetracyle 13. Oxidation and iodination of the enone then gave 3.
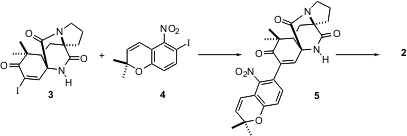
Ullman coupling of 3 with the aryl iodide 4 to give 5 proved to be more
effective than the alternative coupling with the areneboronic acid. Reduction of
5 with activated zinc powder converted the nitro group to the N-OH, which
spontaneously cyclized to the nitrone 2. While 2 so preparared gave a
13C spectrum that was congruent with that of natural avrainvillamide, authentic
material was not available, so a direct comparison could not be made.
In Et3N and CH3CN, 2 spontaneously dimerized to
1. As 2 is levorotatory
(-35°) and 1 is dextrorotatory (91°), this ready interconversion of the monomer
and the dimer will make it difficult to assign the absolute configuration of
either natural product solely by comparison of rotations.