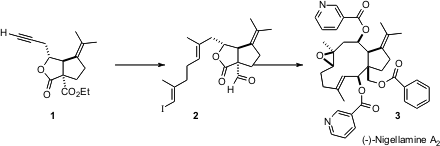
The nigellamine alkaloids, represented by (-)-nigellamine (3), dolabellane diterpenes isolated from black cumin, apparently have lipid metabolism promoting activity. Joseph M. Ready of UT Southwestern Medical Center has described (J. PMID:23514335 Am. Chem. Soc. 2006, 128, 7428.DOI: 10.1021/ja061559n)the first synthesis of a nigellamine, (-)-nigellamine A2 (3). Key steps in the synthesis include an enantioselective cyclization to prepare 1, and the Cr-mediated cyclization of the aldehyde 2.
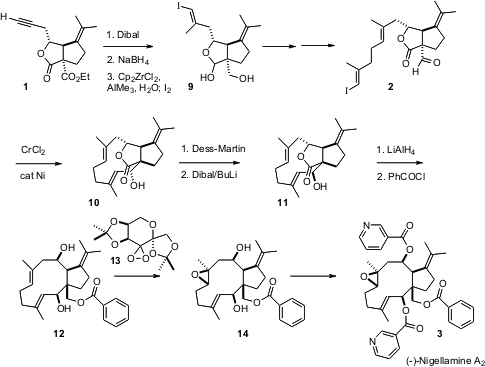
(-)-Nigellamine A2 (3) has an angularly substituted trans ring fusion. 1,7-Naphthyridin-3-amine site The key to the synthesis was the preparation of the angularly-substituted cis-fused lactone 1. The absolute configuration of 1 was set by the Pd-mediated SN2′ cyclization of the malonate 6, which proceeded in 95% ee. 3-(Difluoromethyl)aniline manufacturer Equally important was the seemingly mundane iodine-mediated cyclization of 7 to 8. This one transformation differentiated the two esters of 6, secured the relative configuration of one of the two secondary alcohols of 3, and established the requisite cis ring fusion of the lactone 1. Nucleophilic displacement of the iodide 8 failed, but two-carbon homologation to the alkyne could be accomplished by the free radical Fuchs procedure.
There were intial difficulties with the Negishi methylation/iodination of the terminal alkyne, as the Cp2ZrCl2 reacted preferentially with the lactol. Remarkably, repeating the reaction in the presence of water led to clean addition to the alkyne. Homologation followed by, again, wet Negishi methylation/iodination set the stage for oxidation to the lactone aldehyde 2.
Both the bicyclic skeleton of 2 and the geometry of the two alkenes direct the pendant chain toward the aldehyde. In fact, Ni-catalyzed cyclization proceeded smoothly. The product alcohol emerged as a single diastereomer, but unfortunately the wrong one, so an oxidation/reduction cycle was required to correct the secondary alcohol center.
The final challenge was the selectiveepoxidation of the triene 12. There are two concerns: facial selectivity, and chemoselectivity. The facial selectivity is inherent, as the geometry of the medium ring is such that only the desired face is exposed. Chemoselectivity was more challenging. MCPBA reacted indiscriminantly with each of the three alkenes. Reasoning that a bulkier epoxidizing agent might be more selective, they found that the Shi dioxirane (13) delivered a 7:1 ratio of the two trisubstituted epoxides. It is interesting that the enantiomer of 13 gave only a 2:1 ratio.