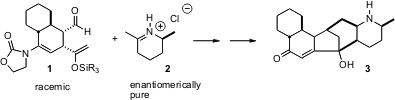
The galbulimima alkaloids have intriguing physiological activity, but the absolute configuration of glabulimima alkaloid 13 (3) had not been assigned. Mohammad Movassaghi of the Massachusetts Institute of Technology has developed (J. Buy1350629-55-8 Am. 4-Aminobutan-1-ol uses Chem. Soc. 2006, 128, 8126.DOI: 10.1021/ja0626180)an elegant route to 3, based on the convergent coupling of racemic 1 with the enantiomerically-pure 2 (for a racemic synthesis of 3:![]() 2004 Feb 23).
2004 Feb 23).
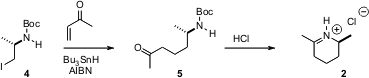
The enantiomerically-pure iodide 4 was prepared from L-alaninol. PMID:23962101 Conjugate addition of the derived radical to methyl vinyl ketone gave 5, which was hydrolyzed to 2.
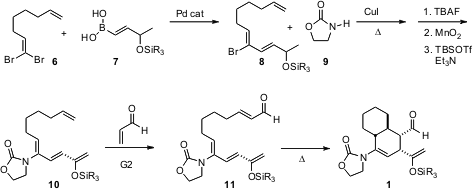
The Diels-Alder substrate 11 was prepared from the 1,1-dibromo alkene6. Pd-mediated coupling of 6 with 7 gave 8. Further coupling with 9 followed by oxidation and enol ether formation gave 10, which underwent smooth cross-coupling with acrolein, yielding 11. The anticipated intramolecularDiels-Alder cycloaddition led to (racemic) 1 with the expected high diastereocontrol.
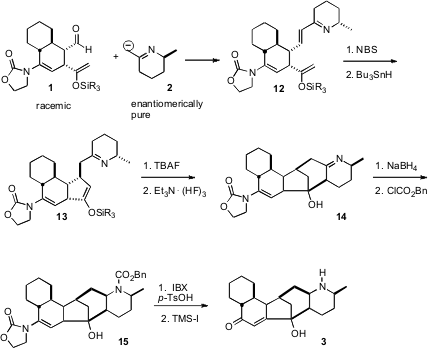
The enantiomerically-pure imine 2 was readily deprotonated, and the resulting anion was condensed with the aldehyde 1 to give, after dehydration, the imine 12. The enol ether was selectively brominated. Free radical reduction led to smooth cyclization, to give 13. On unmasking of the ketone, spontaneous enamine addition ensued, to give the imine14. Stereocontrolled reduction with NaBH4 and protection then gave the pentacylic 15, accompanied by the diastereomer resulting from condensation of 2 with the other enantiomer of 1. These two diastereomers were readily separable by flash chromatography.
To complete the synthesis, the enamide 15 was hydrolyzed by aqueous acid. In situ reduction carried the liberated ketone on to the enone, which was deprotected to give glabulimima alkaloid 13 (3). Both enantiomers of 3 were prepared this way, beginning with each of the enantiomers of 4. The enantiomer derived from L-alaninol gave the same rotation as the natural product, allowing the assignment of the absolute configuration.
The convergent coupling employed here allowed the ready preparation of each of the four enantiomerically-pure diastereomers of the natural product. There are real advantages to such an approach, because it provides not just one substance, the natural product, but each of the four, for further evaluation of physiological activity.