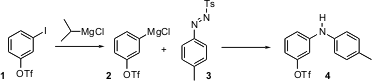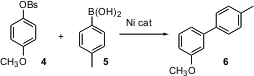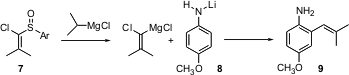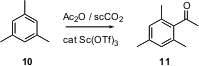The formation of bonds to aromatic rings is the foundation of much of organic synthesis. There has been much excitement over the past several years around Pd- or Cu-mediated displacement of an aromatic halide or aryl sulfonate with an amine. Paul Knochel of the University of Munich reports (Angew. Chem. Int. (5-Bromopyrazin-2-yl)methanol Order Ed. PMID:32695810 2004, 43, 897. DOI: 10.1002/anie.200353241)a complementary approach, the addition of an aryl halide-derived Grignard such as 2 to the diazonium derivative3. Aryl triflates such as 4 are efficient partners for further coupling. 3-Cyclopropyl-1H-1,2,4-triazole site
Although triflates such as 4 participate efficiently in coupling reactions, they are expensive. The attention of several groups has been focused on the development of less expensive alternatives. Qiao-Sheng Hu of CUNY Staten Island recently reported (J. Am. Chem. Soc. 2004, 126, 3058. DOI: 10.1021/ja038752r)that arene tosylates and benzenesulfonates such as 5 can serve as efficient leaving groups for Ni-catalyzed coupling to an areneboronic acid, to give the biaryl 7. While tosylates are commonly used, benzenesulfonyl chloride has advantages on scale, since it is a liquid and so can be metered into a reaction.
Carbon-carbon bond formation ortho to a directing group has a long history in organic synthesis. Tsuyoshi Satoh of the Tokyo University of Science has reported (Tetrahedron Lett. 2004, 45, 5785. DOI: 10.1016/j.tetlet.2004.06.024)a new protocol, based on the reaction of a deprotonated aniline such as 8 with a chloro sulfoxide such as 7. An N-methyl aniline works equally well. The reaction may be proceeding by way of a coordinated alkylidene carbene.
Supercritical CO2 offers many advantages over conventional solvents for organic reactions. The challenge has been to find surfactants that will allow typical organic substrates to dissolve. Shu Kobayashi of the University of Tokyo has described (J. Org. Chem. 2004, 69, 680. DOI: 10.1021/jo0353177)the development of perfluoroalkyl aromatics that serve well, as illustrated by the conversion of 10 to 11. Both the perfluoroalkyl aromatic and the Sc(OTf)3 are recovered at the end of the reaction.