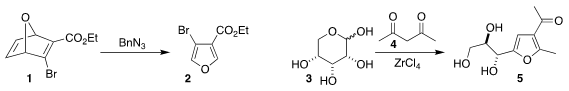
Antonio J. Moreno-Vargas of the Universidad de Sevilla drove the retro
Diels-Alder disassembly of the 7-oxanorbornadiene 1 by reacting it selectively
with benzyl azide, leading to the
furan 2
(J. Org. Chem. 2020, 85, 8923.
DOI: 10.1021/acs.joc.0c00810).
Christopher W. Jones and Stefan France of Georgia Tech prepared the furan 5 by
combining ribose 3 with acetylacetone 4
(J. Org. PMID:36628218 Chem. 2020, 85, 15337.
DOI: 10.1021/acs.joc.0c02176).
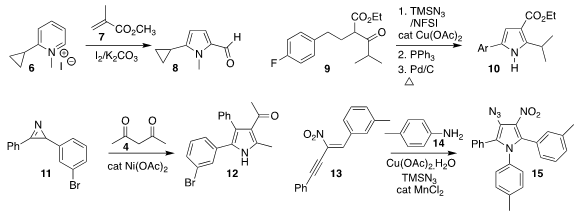
Haiyan Fu of Sichuan University assembled the
pyrrole 8 by reacting the
pyridinium salt 6 with methyl methacrylate 7
(Org. Lett. 2020, 22, 6107.
DOI: 10.1021/acs.orglett.0c02178).
Shannon S. Stahl of the University of Wisconsin effected
benzylic azidation of
9, then cyclized that product to the pyrrole 10
(J. Am. Chem. Soc. 2020, 142, 11388.
DOI: 10.1021/jacs.0c05362).
Mi-Na Zhao of the Baoji University of Arts and Sciences prepared the
pyrrole 12 by adding acetylacetone 4 to the
2H-azirine 11
(Tetrahedron Lett. Buy1217725-33-1 Price of 4-(Dimethylamino)but-2-ynoic acid 2020, 61, 152319.
DOI: 10.1016/j.tetlet.2020.152319).
Haipeng Hu and Peng Cao of Sichuan Normal University reported a parallel investigation
(Org. Chem. Front. 2020, 7, 3686.
DOI: 10.1039/D0QO00951B).
Qiuping Ding of Jiangxi Normal University combined the aniline 14
with the nitroalkene 13 to give the pyrrole 15
(Org. Biomol. Chem. 2020, 18, 8908.
DOI: 10.1039/D0OB01927E).
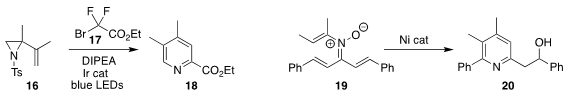
Wen Luo, Jingjing Zhao and Pan Li of Henan University showed that visible
light could promote the coupling of the alkenyl aziridine 16 with the
difluoroacetate 17, leading to the
pyridine 18
(Org. Lett. 2020, 22, 9658.
DOI: 10.1021/acs.orglett.0c03718).
Gui-Fa Su and Dong-Liang Mo of Guangxi Normal University cyclized the N-vinyl
nitrone 19 to the pyridine 20
(Org. Lett. 2020, 22, 8446.
DOI: 10.1021/acs.orglett.0c02947).
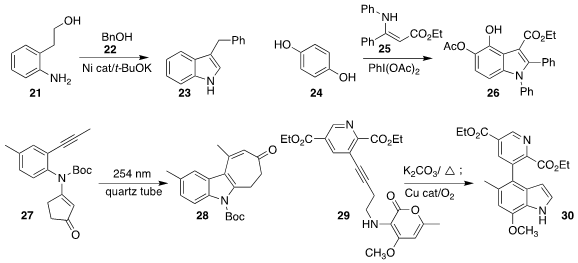
Debashis Adhikari of the Indian Institute of Science Education and Research used a
borrowed hydrogen strategy to combine the aniline 21 with benzyl alcohol 22, leading to the
indole 23
(Chem. Commun. 2020, 56, 15442.
DOI: 10.1039/D0CC07169B).
Jeh-Jeng Wang of Kaohsiung Medical University devised the oxidative coupling of benzhydrol
24 with the enamine 25, leading to the indole 26
(Adv. Synth. Catal. 2020, 362, 2911.
DOI: 10.1002/adsc.202000402).
Martin Hiersemann of TU Dortmund used UV irradiation to convert the
alkyne 27 to the indole 28
(Chem. Eur. J. 2020, 26, 11974.
DOI: 10.1002/chem.202002581).
Christopher M. Beaudry of Oregon State University designed the
Diels-Alder cyclization of the
alkyne 29 to give the indole 30
(Chem. Eur. J. 2020, 26, 16655.
DOI: 10.1002/chem.202004107).
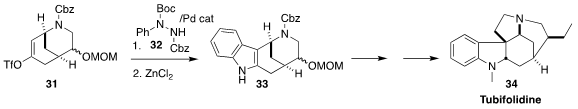
The Strychnos alkaloids, represented by tubifolidine (34), exhibit a wide range
of physiological activites. En route to 34, Cheon-Gyu Cho of Hanyang University
achieved regioselectivity in the
Fischer indole construction of
33 by first
selectively forming the enol triflate 31 from the corresponding ketone, then
coupling that with the protected phenylhydrazine 32, and cyclizing the product
(Org. Lett. 2020, 22, 3464.
DOI: 10.1021/acs.orglett.0c00912).