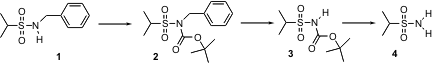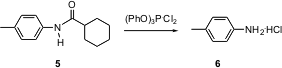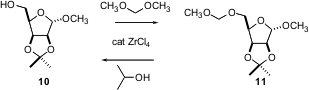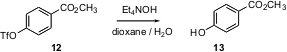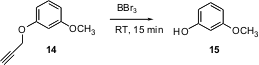O– and N-protection is often necessary in organic synthesis. Several recent advances in functional group protection-deprotection are particularly noteworthy. Formula of (S)-4-(1-Aminoethyl)phenol hydrobromide
Primary benzenesulfonamides have been notoriously difficult to protect. Theodore S. Widlanski of Indiana University has now found (Tetrahedron Lett. 2004, 45, 8483.DOI: 10.1016/j.tetlet.2004.09.118)that whileN-benzyl sulfonamides such as 1 are resistant to hydrogenolytic debenzylation, the easily-prepared Boc derivative 2 is smoothly debenzylated. Brief exposure of 3 to trifluoroacetic acid then gives the primary sulfonamide 4.
Amides are often cleaved with strong alkali. Fabio Prati of the Università di Modena has reported (Org. PMID:24377291 BuyL-Cysteic acid Lett. 2004, 6, 3885.DOI: 10.1021/ol048852h)that treatment of triphenyl phosphite with chlorine at -30°C gives a substance that reacts smoothly with amides such as 5 to give the amine 6 as the HCl salt. The imino chloride is the intermediate, so this also provides a convenient entry to Bischler-Napieralski cyclization.
In addition to the more commonallyl protecting groups, alcohols can be protected as methallyl and prenyl ethers. Pierre Vogel of the Swiss Federal Institute of Technology, Lausanne, has demonstrated (Org. Lett. 2004, 6, 2693. DOI: 10.1021/ol049135q)that using benzenesulfonyl radical, one can efficiently and selectively remove these protecting groups, one after the other.
The MOM ether is a simple protecting group that would be more widely used if it were easier to put on. G.V.M. Sharma of IIT Hyderabad has found (Tetrahedron Lett. 2004, 45, 9229.DOI: 10.1016/j.tetlet.2004.10.091)that the inexpensive ZrCl4 efficiently catalyzes the reaction of dimethoxymethane with an alcohol such as 10 to give the MOM ether11. MOM ethers are easily deprotected with the same catalyst in 2-propanol. Note that both reactions were carried out in the presence of other acid-labile functional groups.
Phenol protection enjoys a special place in organic synthesis. In addition to being good leaving groups for coupling reactions, sulfonates, e.g. a triflate such as 12, are attracting growing attention because of the way they change the reactivity around the arene ring. This necessitates eventual deprotection. Shigeru Nishiyama of Keio University, Yokohama has shown (Tetrahedron Lett. 2004, 45, 6317.DOI: 10.1016/j.tetlet.2004.06.104)that Et4NOH in aqueous dioxane converts arene triflates into the corresponding phenols under mild conditions. Again, note that the easily-hydrolyzed methyl ester survives.
M.G. Finn of Scripps/La Jolla has explored (Org. Lett. 2004, 6, 2777.DOI: 10.1021/ol0489898)propargyl protection for phenols. The propargyl ether is removed by BBr3 more easily than the usually-labile methyl ether. The propargyl group is removed from a primary alcohol under comparable conditions.