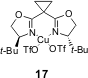Indole Nucleus – Part Two of Two:
Enantioselective Friedel-Crafts reaction
Recently, the catalytic, enantioselective
Friedel-Crafts (FC) alkylation of
indoles has been the method of choice in the synthesis of important optically
active targets. The Jørgensen group was the first to report (Angew. Chem.
Int. Ed. 2001,
40, 160,
DOI: 10.1002/1521-3773(20010105)40:1%3C160::AID-ANIE160%3E3.0.CO;2-S
and Chem. PMID:24318587 Commun. 2001, 347,
DOI: 10.1039/b009008p.)
on the asymmetric FC alkylation of indoles (with α,β-unsaturated ketoesters and
malonates), and many groups have developed different catalytic approaches since
then. Buy(t-Bu)PhCPhos Pd G3 This highlight focuses on the most recent advances in this area.
Part II. Enantioselective Friedel-Crafts Alkylation of the Indole Nucleus
Tang et al. reported (J. Org. Chem. 2004, 69, 1309.
DOI: 10.1021/jo035552p)
on the
enantioselective alkylation of the indole framework with alkylidene malonates
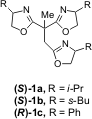
using a complex of the pseudo-C3-symmetric tris(oxazoline) (1) and
copper(II) salts as a catalyst (Figure 1). This group has extensively studied
the solvent influence, in particular the effect of alcohols, tolerance to water,
the influence of the ester group and substituents on the indole, additives,
mechanism and the scope of the reaction.
Figure 1 – Pseudo-C3-symmetric tris(oxazoline) (1)
used for the enantioselective FC alkylation of indole. Formula of 173841-05-9
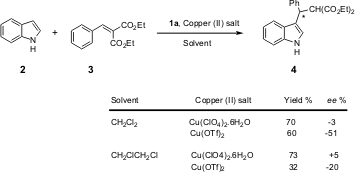
In preliminary tests (Scheme 1), the Tang group observed reversal of
enantioselectivity wherein the stereochemistry of the products was dependent on
the solvent. This gives access to both enantiomers. Another interesting point
was the effect of the alcohol – with 2 equiv. of
1,1,1,3,3,3-hexafluoro-2-propanol, 91% ee and 99% yield were achieved. The
presence of the alcohol not only accelerated the reaction but also improved
enantioselectivity.
Scheme 1 – Preliminary tests where a reverse of
enantioselectivity was observed by Tang et al.
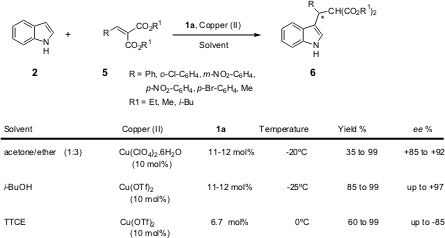
The same group investigated the influence of the reaction conditions
(solvent/ligand/copper salt/temperature) for different arylidene malonates
(Scheme 2). The best enantiomeric excess was obtained in TTCE
(1,1,2,2-tetrachloroethane) with the malonate containing an electron-withdrawing
group on the aromatic ring. A reversal of enantioselectivity was also observed
here: one enantiomer was obtained in TTCE, while in i-BuOH or mixed
solvent (acetone/ether) the opposite enantiomer was formed. The presence of an
aromatic substituent on the double bond is necessary for achieving a significant
enantiomeric excess.
Scheme 2 – Various reaction conditions tested by Tang et al.
Umani-Rochi et al. reported on the catalytic approaches in the
stereoselective FC alkylation of aromatic compounds (Angew. Chem. Int. Ed.
2004,
43, 550.
DOI: 10.1002/anie.200301679).
This group developed (Tetrahedron Lett. 2003, 44, 5843,
DOI: 10.1016/S0040-4039(03)01400-X);
J. Org. Chem. 2004, 69, 7511.
DOI: 10.1021/jo0487202) a
new enantioselective FC-type conjugate addition of indoles to enones catalysed
by a chiral complex derived from [Al(salen)Cl] 7 and lutidine at room
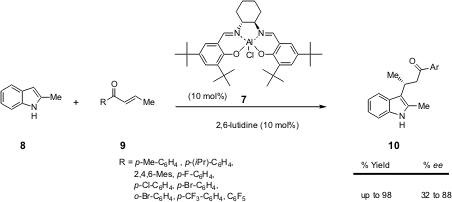
temperature (Scheme 3).
Scheme 3 – Enantioselective FC-type conjugate addition of
indole 8 to (E)-arylcrotyl ketones 9 catalysed by
[Al(salen)Cl] 7 / 2,6-lutidine complex.
Based on the results obtained in their preliminary tests with different
enones and indoles, the Umani-Rochi research group realised that in order to
achieve higher ees, it was essential to have a phenyl group bonded to the
carbonyl moiety and a small methyl group bonded to the double bond, as in enone
9. Enone 9 with R = C6F5 gave the best
result (90% yield, 88% ee) while
9 with R = 2,4,6-Mes (mesityl) or o-Br-C6H5
gave the worst results, probably due to failure to coordinate with the chiral
catalyst.
When the authors screened a variety of solvents (Et2O, THF, MeCN,
CH2Cl2), the best results were obtained with toluene. The
use of 2,6-lutidine (10 mol%) as an additive proved to increase the
stereoselectivity when compared to the reactions carried in the absence of this
base. This group carried out several studies that strongly suggest an
interaction between the added base and the aluminium. Moreover, a cationic
hexacoordinate complex has been proposed as responsible for the observed
enantioselectivity.
García et al reported (J. Am. Chem. Soc. 2005, 127,
4154.
DOI: 10.1021/ja0423217)
very
recent advances in the catalytic enantioselective FC alkylations using a
bis(oxazoline)-Cu(II) as catalyst. This group has performed several reactions
between indoles 14 and α’-hydroxy enones 15, and obtained the
corresponding adducts in high yield and high ee (up to 98%, Scheme 4). These
reactions were carried at temperatures between 0-25ºC as well as at reflux
(40ºC).
Scheme 4 – Enantioselective FC-type conjugate addition of
several indoles
14 to α’-hydroxy enones 15 catalysed by bis(oxazoline)/Cu(OTf)2
13.
However, the adducts resulting from enones 15 (R3 = Me2CH
and
c-C6H11) were obtained in lower yield, although
higher ees and higher yields were achieved when catalyst 17 (Figure 2)
was used (for example: for R3 = c-C6H11,
32% yield with catalyst 13 to 80% yield with catalyst 17).
Figure 2 – Bis(oxazoline)-Cu (II) catalyst 17.