The enantioselective oxygenation procedures, epoxidation and dihydroxylation, developed by Barry Sharpless have dominated single-enantiomer organic synthesis. Recently, several additional methods for enantioselective oxidation have been developed, based on the α-functionalization of carbonyl compounds. 1-(2-Aminoethyl)piperidin-4-ol manufacturer
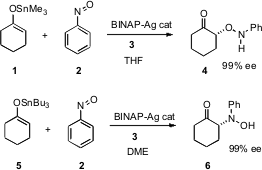
This area is active enough that in two instances the development was reported essentially simultaneously by two different research groups. Hisashi Yamamoto of the University of Chicago (J. Am. Chem. Soc. 2004, 126, 5360. DOI: 10.1021/ja039103i)and Armando Córdova of Stockholm University (Angew. PMID:23847952 Chem. Int. Ed. 2004,43, 1109.DOI: 10.1002/anie.200353018)independently reported the enantioselective α-oxygenation of ketones. 5-(Thiazol-5-yl)nicotinic acid Purity Professor Yamamoto employed Ag-BINAP 3 to catalyze the condensation of a ketone-derived enol ether with nitrosobenzene 2. The trimethylstannyl enol ether 1 led in THF to the α-oxygenated product 4 in high enantiomeric excess. The tributylstannyl enol ether 5 in DME gave the complementary α-aminated product 6, again in excellent ee. Professor Córdova produced 4, also in >99% ee, by condensing cyclohexanone itself with 2, with (S)-proline as the catalyst in CHCl3 or DMSO.
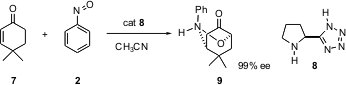
With cyclic enones, Professor Yamamoto has developed (J. Am. Chem. Soc. 2004, 126, 5962.DOI: 10.1021/ja049741g)an enantioselective double functionalization. The organocatalyst 8 mediates conjugate addition of N and α´-oxygenation, to give 9.
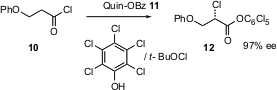
Enantioselective halogenation is a powerful transformation, directly installing an efficient leaving group. Thomas Leckta of Johns Hopkins University has shown (J. Am. Chem. Soc. 2004, 126, 4245.DOI: 10.1021/ja039046t)that benzoylquinine 11 catalyzes the α-chlorination of ketenes derived from acid chlorides such as 10, to give 12 in high ee.
In Communications submitted two weeks apart, David W.C. MacMillan of Caltech (J. Am. Chem. Soc. 2004, 126, 4108.DOI: 10.1021/ja049562z)and Karl Anker Jorgensen of Aarhus University, Denmark (J. Am. Chem. Soc. 2004,126, 4790. DOI: 10.1021/ja049231m)reported the enantioselective α-chlorination of aldehydes, using organocatalysts 14 and 15 respectively. The chloro aldehydes are promising precursors to, inter alia, enantiomerically-pure epoxides.