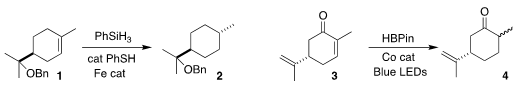
Julian G. West of Rice University observed high diastereoselectivity in the
reduction of the cyclic alkene
1 to 2
(J. Am. Chem. Soc. 2020, 142, 19316.
DOI: 10.1021/jacs.0c09544).
Christopher J. Teskey of RWTH Aachen University selectively reduced carvone 3 to dihydrocarvone 4
(Angew. Chem. 201929-84-2 web Int. PMID:24516446 Ed. 2020, 59, 21176.
DOI: 10.1002/anie.202009893).
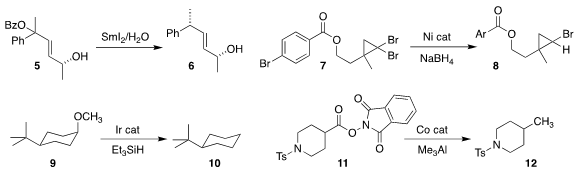
Gregory W. ONeil of Western Washington University also achieved significant diastereoselectivity in the
deoxygenation of the allylic benzoate 5 to 6
(Tetrahedron 2020, 76, 131707.
DOI: 10.1016/j.tet.2020.131707).
Bruce H. Lipshutz of the University of California, Santa Barbara showed that with a limited amount of
NaBH4, the
dibromocyclopropane 7 could be selectively reduced to the monobromocyclopropane 8
(Angew. Chem. Methyltrioxorhenium(VII) site Int. Ed. 2020, 59, 17587.
DOI: 10.1002/anie.202006162).
Nathan D. Schley of Vanderbilit University deoxygenated the axial methyl ether of 9, leading to 10
(ACS Catal. 2020, 10, 6450.
DOI: 10.1021/acscatal.0c01718).
Rui Shang of the University of Tokyo and Yao Fu of the University of Science and Technology of China
replaced the activated ester of 11 with a methyl group, leading to 12
(Synlett 2020, 31, 1221.
DOI: 10.1055/s-0040-1707946).
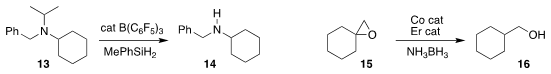
Sukbok Chang of KAIST prepared the secondary amine 14 by
dealkylating the tertiary amine 13
(J. Am. Chem. Soc. 2020, 142, 12585.
DOI: 10.1021/jacs.0c05241).
Thomas Werner of the Leibniz Institute for Catalysis, Rostock observed that the
reduction of the epoxide 15
to the primary alcohol 16 proceeded via initial rearrangement to the aldehyde
(ACS Catal. 2020, 10, 13659.
DOI: 10.1021/acscatal.0c03294).
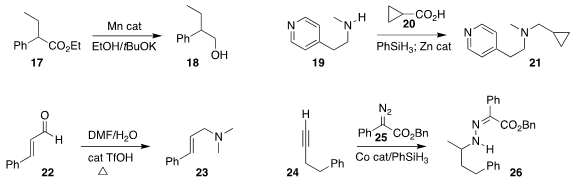
Matthew L. Clarke of the University of St. Andrews used a Mn catalyst to
effect the transfer
hydrogenation of the ester
17 to the primary alcohol 18
(Chem. Commun. 2020, 56, 8635.
DOI: 10.1039/D0CC02598D).
Ross M. Denton of the University of Nottingham used the carboxylic acid 20 under reducing
conditions to alkylate the secondary amine 19, leading to the tertiary amine 21
(Chem. Sci. 2020, 11, 9494.
DOI: 10.1039/D0SC02271C).
Thanh Vinh Nguyen of the University of New South Wales used dimethylformamide to
convert the aldehyde 22 to the tertiary amine 23
(Chem. Commun. 2020, 56, 8691.
DOI: 10.1039/D0CC02955F).
Zhan Lu of Zhejiang University constructed the
hydrazone 26 by adding the diazo
ester 25 to the alkyne 24 under reducing conditions
(J. Am. Chem. Soc. 2020, 142, 14455.
DOI: 10.1021/jacs.0c07258).
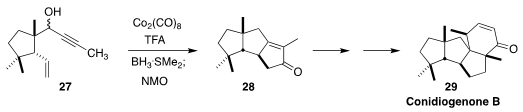
Conidiogenone B (29), isolated from Penicillium, showed significant activity
against methicillin-resistant Staphylococcus aureus. In the course of assembling
29, Hongbin Zhai of the Shenzen Graduate School of Peking University effected
the tandem Nicholas reduction/Pauson-Khand cyclization of the
propargylic alcohol 27 to the cyclopentenone 28
(Angew. Chem. Int. Ed. 2020, 59, 16475.
DOI: 10.1002/anie.202007247).
Jack Dunitz of ETH passed away this last week. In many ways, he opened the path for all of computational organic chemistry.