In the last few years, chiral sulfoxides have become important targets in organic synthesis, and their chemistry has recently been reviewed (Org. Chem. Highlights 2004, December 24. Link). An increasing number of applications of these compounds are evident, from chiral auxiliaries to pharmaceuticals. This is mainly due to new developments in the enantioselective sulfoxidation of sulfides that is the most straightforward method for their synthesis, where overoxidation is the major problem. The methods available are quite extensive (Tetrahedron2005, 61, 1933. DOI: 10.1016/j.tet.2005.05.044; Tetrahedron 2005, 61, 8315. DOI: 10.1016/j.tet.2004.11.041), although there is still a significant need for enantioselective conversions. The following examples illustrate the potential of this methodology.
This Highlight covers important developments in this field from 2004 to present. The chiral-switch concept is strongly related to this topic, and an excellent review was published by Caldwell and co-workers (Nature Reviews Drug Discovery 2002, 1, 753. DOI: 10.1038/nrd915).
1. Chemical oxidation
The orally active HIV-1 therapeutic agent candidate 1, was synthesized by Tawada and co-workers via the enantioselective sulfoxidation of a sulfide intermediate (72% yield, 98% ee) (Tetrahedron 2005, 61, 5043. DOI: 10.1016/j.tet.2005.03.036).
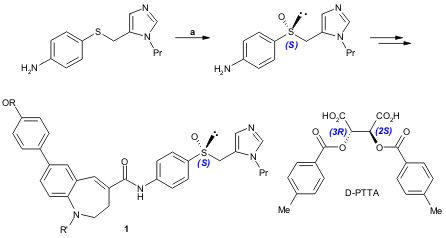
a) substrate (1.0 equiv.), D-PTTA (1.0 equiv.), H2O2 30% (4.0 equiv.), r.t., 3 weeks. D-PTTA= Di-p-toluoyl-D-tartaric acid. 1245647-53-3 Chemscene
Lewis acid-mediated activation of chiral N-alkyloxaziridines 2 was investigated by Fontecave and co-workers in the enantioselective oxidation of sulfides. PMID:23509865 Using heteroaromatic substitutedoxaziridines and ZnCl2, an oxaziridinium salt intermediate is formed and a fast, efficient (23-85% yield) and selective (22-63% ee) oxygen transfer to sulfides is observed (J. Org. Chem. 2005, 70, 301. DOI: 10.1021/jo048380k). 6-Bromo-4(1H)-cinnolinone Order
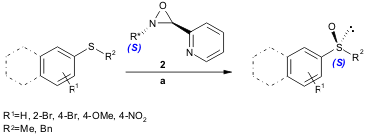
a) oxaziridine 2/ZnCl2/substrate ratio, 1.2:1.2:1, CHCl3, several min.
Vanadium complexes of chiral Schiff bases 3, reported by Ahn and co-workers, were found to catalyze the enantioselective oxidation of alkyl aryl (76-95% yield, 31-87% ee) and benzyl aryl (67-90% yield, 67-99% ee) sulfides (Tetrahedron Lett. 2004, 45, 9249. DOI: 10.1016/j.tetlet.2004.10.070).
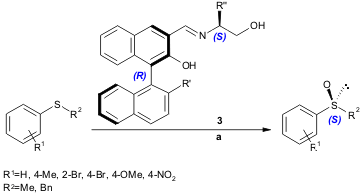
a) ligand 3 (1.5-3 mol%), VO(acac)2, (1-2 mol%), H2O2 35% (1.1 equiv.), substrate (1.0 equiv.), CH2Cl2, 0 ºC, 24 h. acac= acetylacetonate.
A similar approach was made by Sartorio and co-workers, who used polymer-supported chiral Schiff bases 4. The catalytic (up to four cycles) enantioselective oxidation proceeds in good yield (54-80%) and selectivity (45-57% ee) (Tetrahedron: Asymmetry, 2004, 15, 2467. DOI: 10.1016/j.tetasy.2004.06.041).
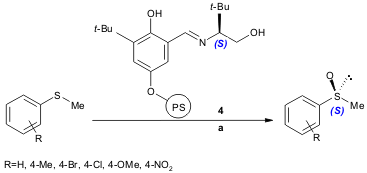
a) ligand 4/VO(acac)2 (1mol%), substrate (1.0 equiv.), H2O2 30% (1.1 equiv.), CH2Cl2/MeOH (250:1), 0 ºC, 16 h. acac= acetylacetonate.
Strukul and Scarso report a simple, mild, catalytic enantioselective (up to 88% ee) oxidation of prochiral aryl alkyl sulfides. This high-yielding (63-99%) methodology is mediated by a chiral platinum diphosphine complex 5 and can be performed in a water-surfactant medium (Adv. Synth. Catal. 2005, 347, 1227. DOI: 10.1002/adsc.200505030). For recent developments of organic synthesis in aqueous medium, see a previous Highlight (Org. Chem. Highlights 2005, July 25. Link).
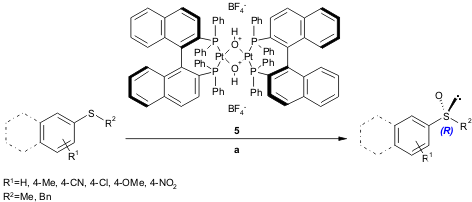
a) catalyst 5/H2O2 35%/substrate ratio, 1:100:100, H2O/SDS (1 mM in micelles), r.t., 8.5-48 h.
A new modification of the Modena protocol [Ti(Oi-Pr)4/(R,R)-DET 1:4 and t-butyl hydroperoxide] for the enantioselective oxidation of alkyl (73% yield, 91% ee) and alkyl aryl (63-99% yield, 70-97% ee) sulfides is now reported by Scettri and co-workers. Their reinvestigation of this protocol led the authors to achieve a catalytic, highly enantioselective procedure using furyl (6) or cumyl hydroperoxides (7). This modification was also successfully employed in the mono-sulfoxidation of 8 and other 2-aryl substituted 1,3-dithiolanes (Tetrahedron: Asymmetry 2005, 16, 2271. DOI: 10.1016/j.tetasy.2005.05.015).
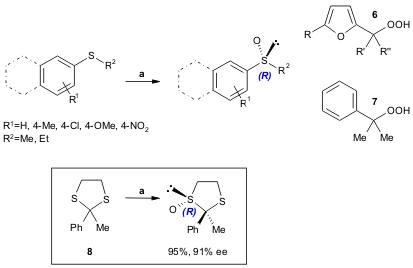
a) oxidant 6 or 7 (1.2 equiv.), Ti(Oi-Pr)4/(R,R)-DET 1:4 (1.0 equiv.), substrate (1.0 equiv.), CH2Cl2, -20 ºC, 6-72 h. DET= (R,R)-diethyl tartrate.
2. Chemoenzymic oxidation
A series of Baeyer-Villiger monooxygenases (BVMOs): phenylacetone monooxygenase (PAMO) from Thermobifida fusca, 4-hydroxyacetophenone monooxygenase (HAPMO) from Pseudomonas fluorescens ACB, cyclohexanone monooxygenase (CHMO) from Acinetobacter NCIMB 9871 and ethionamide monooxygenase (EtaA) from Mycobacterium tuberculosis, were found to oxidize alkyl aryl sulfides with high enantiomeric excess in the presence of the organorhodium complex 9. Based on these results, Fraaije and co-workers believe that this system is able to replace coenzyme regeneration (Table 1) (Chem. Commun. 2005, 3724. DOI: 10.1039/b504921k).
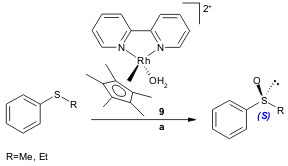
a) catalyst 9 (1.0 mmol/L), enzyme (1.0 U), NADPH+ (1.3 mmol/L), potassium phosphate buffer (pH 9.0)/sodium formate (0.1 M), substrate (16 mmol/L), 250 rpm, 25 ºC, 16-24 h.

3. Enzymic oxidation
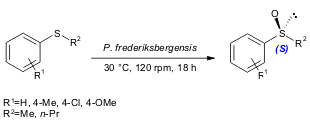
Schreier and co-workers discovered that the commercially available topsoil bacterium Pseudomonas frederiksbergensis DSM 13022 is able to oxidize alkyl (98% yield, 70% ee) and alkyl aryl sulfides (27-100% yield, 81-99% ee) using a whole-cell system (Tetrahedron: Asymmetry 2004, 15, 983. DOI: 10.1016/j.tetasy.2003.12.030).
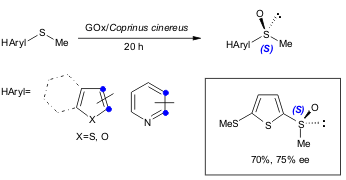
An oxidase/peroxidase bienzymic system developed by Therisod and co-workers was tested in the oxidation of several alkyl heteroaryl sulfides (40-100% yield, 41-99% ee). The protocol uses cheap, commercially available enzymes and can be performed in large scale synthesis. The authors found also that mono-sulfoxidation occurs with good enantioselectivity and that some substrates are insensitive to oxidation under these conditions (Tetrahedron: Asymmetry2005, 16, 2681. DOI: 10.1016/j.tetasy.2005.07.004).