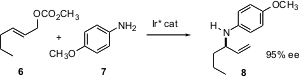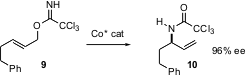The enantioselective construction of aminated stereogenic centers is a central task both for pharmaceutical production and for alkaloid synthesis.
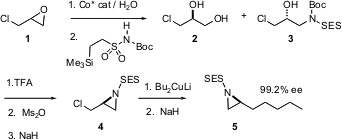
Activated aziridines should be as useful as epoxides for carbon-carbon bond formation, with the advantage that the product will already incorporated the desired secondary aminated stereocenter. To date, a general enantioselective method for the aziridination of alkenes has not been developed. PMID:24428212 Methyl 6-(chloromethyl)picolinate uses Eric Jacobsen of Harvard University (Angew. Chem. Int. Buy30132-23-1 Ed. 2004, 43, 3952.DOI: 10.1002/anie.200460369)has explored an interim solution, based on the resolution of racemic epoxides such as 1. The cobalt catalyst that selectively hydrolyzes one enantiomer of the epoxide also promotes the addition of the imide to the remaining enantiomerically-enriched epoxide. As expected, the aziridine 4 is opened smoothly with dialkyl cuprates.
Two methods for the direct construction of enantiomerically-enriched allylic amines have recently been reported. John Hartwig of Yale University has developed (Angew. Chem. Int. Ed. 2004, 43, 4797.DOI: 10.1002/anie.200460276)an Ir complex that effects the coupling of allylic carbonates such as 6 with aromatic amines to give the secondary amine in high ee.
In a complementary approach, Larry Overman of the University of California at Irvine, has developed (J. Org. Chem. 2004, 69, 8101.DOI: 10.1021/jo0487092)a Co catalyst that effects the rearrangement of allylic imidates such as 9 with high ee. There is no need for the starting allylic alcohols to be perfectlytrans, as imidates from cis allylic alcohols do not participate in the rearrangement.
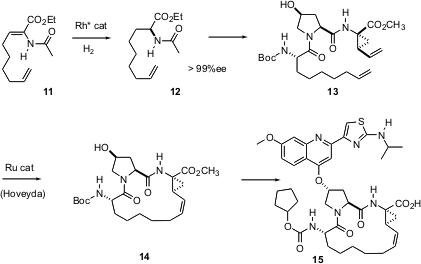
The first-developed method for the enantioselective construction of secondary aminated stereocenters, by Knowles, was the hydrogenation of enamides such as 11. In the context of a synthesis of BILN 2061 (15), an antiviral protease inhibitor, Anne-Marie Faucher of Boehringer-Ingelheim, Laval has shown (Org. Lett. 2004, 6, 2901.DOI: 10.1021/ol0489907)that such hydrogenations can be effected even in the presence of a terminal vinyl group. The product 12 was carried on to 15 over several steps, including the Ru (Hoyveda) cyclization of 13 to 14.